1. Zacharowski K, Spahn DR. Patient blood management equals patient safety. Best Pract Res Clin Anaesthesiol. 2016; 30:159–69.

2. Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Meyer W, Scalea TM. Outcome analysis of blood product transfusion in trauma patients: a prospective, risk-adjusted study. World J Surg. 2008; 32:2185–9.

3. Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013; 96:478–85.

4. Turan A, Yang D, Bonilla A, Shiba A, Sessler DI, Saager L, et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anaesth. 2013; 60:761–70.

5. Ruseckaite R, McQuilten ZK, Oldroyd JC, Richter TH, Cameron PA, Isbister JP, et al. Descriptive characteristics and in-hospital mortality of critically bleeding patients requiring massive transfusion: results from the Australian and New Zealand Massive Transfusion Registry. Vox Sang. 2017; 112:240–8.

6. Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PG, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010; 210:957–65.

7. Desborough M, Sandu R, Brunskill SJ, Doree C, Trivella M, Montedori A, et al. Fresh frozen plasma for cardiovascular surgery. Cochrane Database Syst Rev. 2015; (7):CD007614.

8. Baharoglu MI, Cordonnier C, Salman RA, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016; 387:2605–13.

9. Warner MA, Chandran A, Jenkins G, Kor DJ. Prophylactic plasma transfusion is not associated with decreased red blood cell requirements in critically Ill patients. Anesth Analg. 2017; 124:1636–43.

10. Warner MA, Jia Q, Clifford L, Wilson G, Brown MJ, Hanson AC, et al. Preoperative platelet transfusions and perioperative red blood cell requirements in patients with thrombocytopenia undergoing noncardiac surgery. Transfusion. 2016; 56:682–90.

11. Bolton-Maggs PH, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013; 163:303–14.
12. Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, et al. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg. 2014; 76:561–7.

13. Khan S, Davenport R, Raza I, Glasgow S, De’Ath HD, Johansson PI, et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med. 2015; 41:239–47.

14. Balvers K, van Dieren S, Baksaas-Aasen K, Gaarder C, Brohi K, Eaglestone S, et al. Combined effect of therapeutic strategies for bleeding injury on early survival, transfusion needs and correction of coagulopathy. Br J Surg. 2017; 104:222–9.

15. Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015; 313:471–82.
16. Cannon JW, Johnson MA, Caskey RC, Borgman MA, Neff LP. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J Trauma Acute Care Surg. 2017; 83:211–7.

17. McQuilten ZK, Crighton G, Brunskill S, Morison JK, Richter TH, Waters N, et al. Optimal dose, timing and ratio of blood products in massive transfusion: results from a systematic review. Transfus Med Rev. 2018; 32:6–15.

18. Winearls J, Reade M, Miles H, Bulmer A, Campbell D, Görlinger K, et al. Targeted coagulation management in severe trauma: the controversiesand the evidence. Anesth Analg. 2016; 123:910–24.
19. Toulon P, Ozier Y, Ankri A, Fléron MH, Leroux G, Samama CM. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost. 2009; 101:394–401.
20. Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011; 39:2652–8.

21. Olde Engberink RH, Kuiper GJ, Wetzels RJ, Nelemans PJ, Lance MD, Beckers EA, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014; 28:210–6.

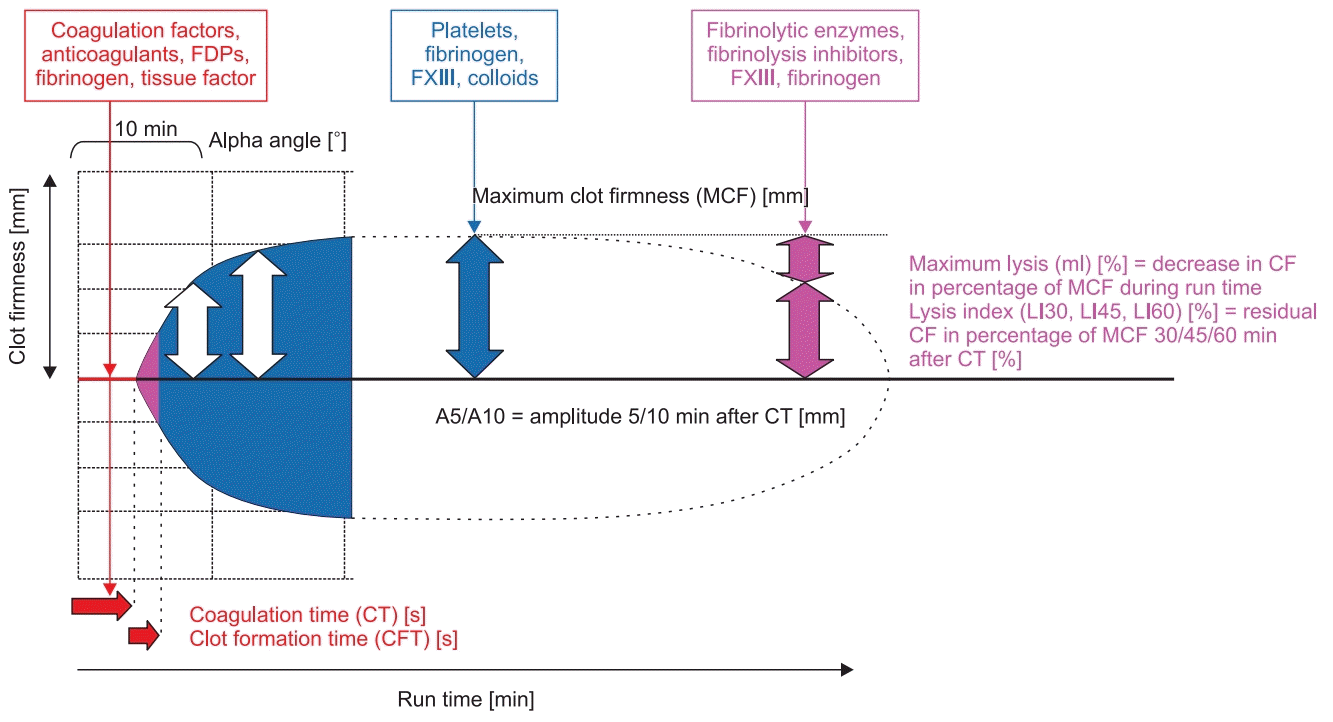
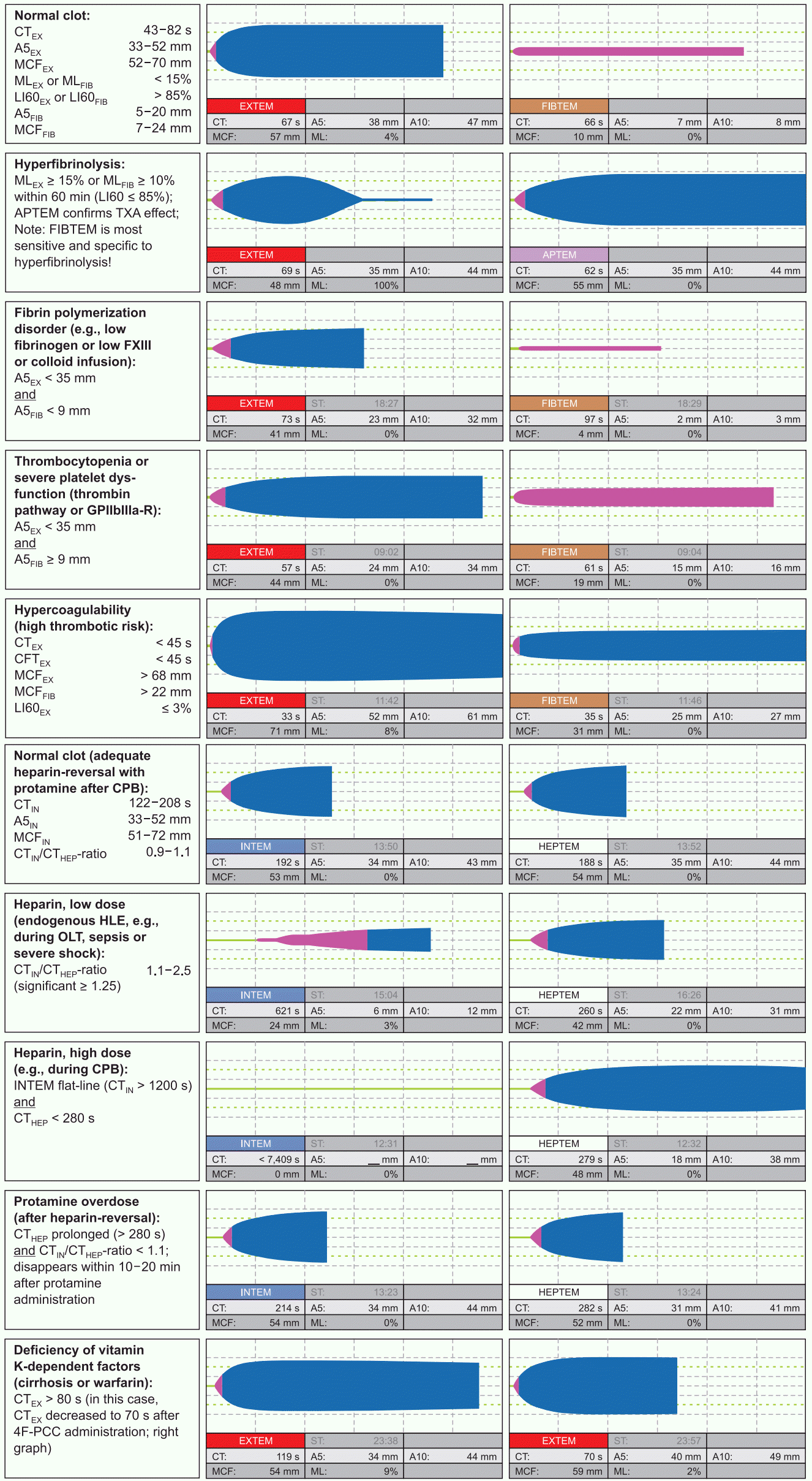
22. Görlinger K, Dirkmann D, Solomon C, Hanke AA. Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br J Anaesth. 2013; 110:222–30.

23. Dirkmann D, Görlinger K, Dusse F, Kottenberg E, Peters J. Early thromboelastometric variables reliably predict maximum clot firmness in patients undergoing cardiac surgery: a step towards earlier decision making. Acta Anaesthesiol Scand. 2013; 57:594–603.

24. Perez-Ferrer A, Vicente-Sanchez J, Carceles-Baron MD, Van der Linden P3, Faraoni D. Early thromboelastometry variables predict maximum clot firmness in children undergoing cardiac and non-cardiac surgery. Br J Anaesth. 2015; 115:896–902.

25. Song JG, Jeong SM, Jun IG, Lee HM, Hwang GS. Five-minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantation. Br J Anaesth. 2014; 112:290–7.

26. Toffaletti JG, Buckner KA. Use of earlier-reported rotational thromboelastometry parameters to evaluate clotting status, fibrinogen, and platelet activities in postpartum hemorrhage compared to surgery and intensive care patients. Anesth Analg. 2019; 128:414–23.

27. Schöchl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care. 2011; 15:R265.
28. Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care. 2015; 19:97.

29. Na HS, Shin HJ, Do SH. FIBTEM provides prediction of massive bleeding in total hip replacement arthroplasty. Blood Coagul Fibrinolysis. 2016; 27:340–6.

30. Collins PW, Lilley G, Bruynseels D, Laurent DB, Cannings-John R, Precious E, et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: a prospective study. Blood. 2014; 124:1727–36.

31. Dötsch TM, Dirkmann D, Bezinover D, Hartmann M, Treckmann JW, Paul A, et al. Assessment of standard laboratory tests and rotational thromboelastometry for the prediction of postoperative bleeding in liver transplantation. Br J Anaesth. 2017; 119:402–10.

32. Weber CF, Görlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012; 117:531–47.
33. Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015; 19:1–228.

34. Deppe AC, Weber C, Zimmermann J, Kuhn EW, Slottosch I, Liakopoulos OJ, et al. Point-of-care thromboelastography/thromboelastometrybased coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res. 2016; 203:424–33.
35. Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016; (8):CD007871.

36. Brohi K, Eaglestone S. Traumatic coagulopathy and massive transfusion: improving outcomes and saving blood. Programme Grants Appl Res. 2017; 5:1–73.

37. Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005; 16:301–10.

38. Schenk B, Görlinger K, Treml B, Tauber H, Fries D, Niederwanger C, et al. A comparison of the new ROTEMⓇ sigma with its predecessor, the ROTEMdelta. Anaesthesia. 2019; 74:348–56.
39. Oswald E, Stalzer B, Heitz E, Weiss M, Schmugge M, Strasak A, et al. Thromboelastometry (ROTEM) in children: age-related reference ranges and correlations with standard coagulation tests. Br J Anaesth. 2010; 105:827–35.
40. Sokou R, Foudoulaki-Paparizos L, Lytras T, Konstantinidi A, Theodoraki M, Lambadaridis I, et al. Reference ranges of thromboelastometry in healthy full-term and pre-term neonates. Clin Chem Lab Med. 2017; 55:1592–7.

41. de Lange NM, van Rheenen-Flach LE, Lancé MD, Mooyman L, Woiski M, van Pampus EC, et al. Peri-partum reference ranges for ROTEM(R) thromboelastometry. Br J Anaesth. 2014; 112:852–9.
42. Oudghiri M, Keita H, Kouamou E, Boutonnet M, Orsini M, Desconclois C, et al. Reference values for rotation thromboelastometry (ROTEMⓇ) parameters following non-haemorrhagic deliveries. Correlations with standard haemostasis parameters. Thromb Haemost. 2011; 106:176–8.

43. Blasi A, Beltran J, Pereira A, Martinez-Palli G, Torrents A, Balust J, et al. An assessment of thromboelastometry to monitor blood coagulation and guide transfusion support in liver transplantation. Transfusion. 2012; 52:1989–98.

44. Fayed N, Mourad W, Yassen K, Görlinger K. Preoperative thromboelastometry as a predictor of transfusion requirements during adult living donor liver transplantation. Transfus Med Hemother. 2015; 42:99–108.

45. Nakayama Y, Nakajima Y, Tanaka KA, Sessler DI, Maeda S, Iida J, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth. 2015; 114:91–102.

46. Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011; 115:1179–91.
47. Petricevic M, Konosic S, Biocina B, Dirkmann D, White A, Mihaljevic MZ, et al. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM(Ⓡ) platelet and Multiplate(Ⓡ) impedance aggregometry. Anaesthesia. 2016; 71:636–47.
48. Faraoni D, Emani S, Halpin E, Bernier R, Emani SM, DiNardo JA, et al. Relationship between transfusion of blood products and the incidence of thrombotic complications in neonates and infants undergoing cardiacsurgery. J Cardiothorac Vasc Anesth. 2017; 31:1943–8.
49. Görlinger K, Iqbal J, Dirkmann D, Tanaka KA. Whole blood assay: thromboelastometry. In: Management of Bleeding Patients. Teruya J, editor. Basel: Springer Nature Switzerland AG;2016. p. 37–64.
50. Gronchi F, Perret A, Ferrari E, Marcucci CM, Flèche J, Crosset M, et al. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in-vivo study. Eur J Anaesthesiol. 2014; 31:68–75.
51. Ortmann E, Rubino A, Altemimi B, Collier T, Besser MW, Klein AA. Validation of viscoelastic coagulation tests during cardiopulmonary bypass. J Thromb Haemost. 2015; 13:1207–16.

52. Mace H, Lightfoot N, McCluskey S, Selby R, Roy D, Timoumi T, et al. Validity of thromboelastometry for rapid assessment of fibrinogen levels in heparinized samples during cardiac surgery: a retrospective, single-center, observational study. J Cardiothorac Vasc Anesth. 2016; 30:90–5.

53. Görlinger K, Shore-Lesserson L, Dirkmann D, Hanke AA, Rahe-Meyer N, Tanaka KA. Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth. 2013; 27(Suppl 4):S20–34.

54. Karkouti K, McCluskey SA, Callum J, Freedman J, Selby R, Timoumi T, et al. Evaluation of a novel transfusion algorithm employing pointof-care coagulation assays in cardiac surgery: a retrospective cohort study with interrupted time-series analysis. Anesthesiology. 2015; 122:560–70.
55. Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedgeclustered randomized controlled trial. Circulation. 2016; 134:1152–62.
56. Ichikawa J, Kodaka M, Nishiyama K, Hirasaki Y, Ozaki M, Komori M. Reappearance of circulating heparin in whole blood heparin concentration-based management does not correlate with postoperative bleeding after cardiac surgery. J Cardiothorac Vasc Anesth. 2014; 28:1003–7.

57. Ni Ainle F, Preston RJ, Jenkins PV, Nel HJ, Johnson JA, Smith OP, et al. Protamine sulfate down-regulates thrombin generation by inhibiting factor V activation. Blood. 2009; 114:1658–65.

58. Mittermayr M, Velik-Salchner C, Stalzer B, Margreiter J, Klingler A, Streif W, et al. Detection of protamine and heparin after termination of cardiopulmonary bypass by thrombelastometry (ROTEM): results of a pilot study. Anesth Analg. 2009; 108:743–50.

59. Gertler R, Wiesner G, Tassani-Prell P, Braun SL, Martin K. Are the point-of-care diagnostics MULTIPLATE and ROTEM valid in the setting of high concentrations of heparin and its reversal with protamine? J Cardiothorac Vasc Anesth. 2011; 25:981–6.

60. Ortmann E, Klein AA, Sharples LD, Walsh R, Jenkins DP, Luddington RJ, et al. Point-of-care assessment of hypothermia and protamine-induced platelet dysfunction with multiple electrode aggregometry (MultiplateⓇ) in patients undergoing cardiopulmonary bypass. Anesth Analg. 2013; 116:533–40.

61. Koster A, Börgermann J, Gummert J, Rudloff M, Zittermann A, Schirmer U. Protamine overdose and its impact on coagulation, bleeding, and transfusions after cardiopulmonary bypass: results of a randomized double-blind controlled pilot study. Clin Appl Thromb Hemost. 2014; 20:290–5.
62. Meesters MI, Veerhoek D, de Lange F, de Vries JW, de Jong JR, Romijn JW, et al. Effect of high or low protamine dosing on postoperative bleeding following heparin anticoagulation in cardiac surgery. A randomised clinical trial. Thromb Haemost. 2016; 116:251–61.
63. Yamamoto T, Wolf HG, Sinzobahamvya N, Asfour B, Hraska V, Schindler E. Prolonged activated clotting time after protamine administration does not indicate residual heparinization after cardiopulmonary bypass in pediatric open heart surgery. Thorac Cardiovasc Surg. 2015; 63:397–403.

64. Karkouti K, Callum J, Crowther MA, McCluskey SA, Pendergrast J, Tait G, et al. The relationship between fibrinogen levels after cardiopulmonary bypass and large volume red cell transfusion in cardiac surgery: an observational study. Anesth Analg. 2013; 117:14–22.
65. Reinhöfer M, Brauer M, Franke U, Barz D, Marx G, Lösche W. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2008; 19:212–9.

66. Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A, et al. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015; 4:e002066.

67. Ranucci M, Baryshnikova E. Fibrinogen supplementation after cardiac surgery: insights from the Zero-Plasma trial (ZEPLAST). Br J Anaesth. 2016; 116:618–23.

68. Ranucci M, Pistuddi V, Baryshnikova E, Colella D, Bianchi P. Fibrinogen levels after cardiac surgical procedures: association with postoperative bleeding, trigger values, and target values. Ann Thorac Surg. 2016; 102:78–85.

69. Ranucci M, Baryshnikova E, Pistuddi V, Menicanti L, Frigiola A. The effectiveness of 10 years of interventions to control postoperative bleeding in adult cardiac surgery. Interact Cardiovasc Thorac Surg. 2017; 24:196–202.

70. Ranucci M, Baryshnikova E, Ranucci M, Silvetti S. Fibrinogen levels compensation of thrombocytopenia-induced bleeding following cardiac surgery. Int J Cardiol. 2017; 249:96–100.

71. Tanaka KA, Bader SO, Görlinger K. Novel approaches in management of perioperative coagulopathy. Curr Opin Anaesthesiol. 2014; 27:72–80.

72. Collins PW, Solomon C, Sutor K, Crispin D, Hochleitner G, Rizoli S, et al. Theoretical modelling of fibrinogen supplementation with therapeutic plasma, cryoprecipitate, or fibrinogen concentrate. Br J Anaesth. 2014; 113:585–95.

73. Flisberg P1, Rundgren M, Engström M. The effects of platelet transfusions evaluated using rotational thromboelastometry. Anesth Analg. 2009; 108:1430–2.

74. Tripodi A, Primignani M, Chantarangkul V, Lemma L, Jovani M, Rebulla P, et al. Global hemostasis tests in patients with cirrhosis before and after prophylactic platelet transfusion. Liver Int. 2013; 33:362–7.

75. Kander T, Tanaka KA, Norström E, Persson J, Schött U. The effect and duration of prophylactic platelet transfusions before insertion of a central venous catheter in patients with bone marrow failure evaluated with point-of-care methods and flow cytometry. Anesth Analg. 2014; 119:882–90.

76. Görlinger K, Jambor C, Hanke AA, Dirkmann D, Adamzik M, Hartmann M, et al. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus Med Hemother. 2007; 34:396–411.

77. Karon BS, Tolan NV, Koch CD, Wockenfus AM, Miller RS, Lingineni RK, et al. Precision and reliability of 5 platelet function tests in healthy volunteers and donors on daily antiplatelet agent therapy. Clin Chem. 2014; 60:1524–31.

78. Scharf RE. Drugs that affect platelet function. Semin Thromb Hemost. 2012; 38:865–83.

79. Ranucci M, Baryshnikova E, Soro G, Ballotta A, De Benedetti D, Conti D. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg. 2011; 91:123–9.

80. Ranucci M, Colella D, Baryshnikova E, Di Dedda U. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth. 2014; 113:970–6.

81. Polzin A, Helten C, Dannenberg L, Mourikis P, Naguib D, Achilles A, et al. Platelet reactivity in patients on aspirin and clopidogrel therapy measured by a new bedside whole-blood assay. J Cardiovasc Pharmacol. 2019; 73:40–7.

82. Mahla E, Suarez TA, Bliden KP, Rehak P, Metzler H, Sequeira AJ, et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ Cardiovasc Interv. 2012; 5:261–9.

83. Romlin BS, Söderlund F, Wåhlander H, Nilsson B, Baghaei F, Jeppsson A. Platelet count and function in paediatric cardiac surgery: a prospective observational study. Br J Anaesth. 2014; 113:847–54.

84. Romlin BS, Söderlund F, Wåhlander H, Hallhagen S, Wessman C, Baghaei F, et al. Perioperative monitoring of platelet function in paediatric cardiac surgery by thromboelastometry, or platelet aggregometry? Br J Anaesth. 2016; 116:822–8.
85. Petricevic M, Milicic D, White A, Boban M, Mihaljevic MZ, Piljic D, et al. Development of a concept for a personalized approach in the perioperative antiplatelet therapy administration/discontinuation management based on multiple electrode aggregometry in patients undergoing coronary artery surgery. J Thromb Thrombolysis. 2015; 40:383–91.

86. Corredor C, Wasowicz M, Karkouti K, Sharma V. The role of point-of-care platelet function testing in predicting postoperative bleeding following cardiac surgery: a systematic review and meta-analysis. Anaesthesia. 2015; 70:715–31.

87. Blasi A, Muñoz G, de Soto I, Mellado R, Taura P, Rios J, et al. Reliability of thromboelastometry for detecting the safe coagulation threshold in patients taking acenocoumarol after elective heart valve replacement. Thromb Res. 2015; 136:669–72.

88. Schmidt DE, Holmström M, Majeed A, Näslin D, Wallén H, Ågren A. Detection of elevated INR by thromboelastometry and thromboelastography in warfarin treated patients and healthy controls. Thromb Res. 2015; 135:1007–11.

89. Dunham CM, Rabel C, Hileman BM, Schiraldi J, Chance EA, Shima MT, et al. TEGⓇ and RapidTEGⓇ are unreliable for detecting warfarincoagulopathy: a prospective cohort study. Thromb J. 2014; 12:4.

90. Hanke AA, Joch C, Görlinger K. Long-term safety and efficacy of a pasteurized nanofiltrated prothrombin complex concentrate (Beriplex P/N): a pharmacovigilance study. Br J Anaesth. 2013; 110:764–72.

91. Grottke O, Levy JH. Prothrombin complex concentrates in trauma and perioperative bleeding. Anesthesiology. 2015; 122:923–31.

92. Görlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schöchl H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother. 2012; 39:104–13.

93. Refaai MA, Goldstein JN, Lee ML, Durn BL, Milling TJ Jr, Sarode R. Increased risk of volume overload with plasma compared with fourfactor prothrombin complex concentrate for urgent vitamin K antagonist reversal. Transfusion. 2015; 55:2722–9.

94. Sarode R, Milling TJ Jr, Refaai MA, Mangione A, Schneider A, Durn BL, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013; 128:1234–43.
95. Clifford L, Jia Q, Subramanian A, Yadav H, Schroeder DR, Kor DJ. Risk factors and clinical outcomes associated with perioperativetransfusion-associated circulatory overload. Anesthesiology. 2017; 126:409–18.
96. Ahn Y, Goerlinger K. Coagulopathy and hypercoagulability. In: Critical Care Handbook of the General Massachusetts Hospital. 6th ed. Wiener-Kronish JP, Bachi A, Chamin JE, Cobb JP, Eikermann M, Quraishi SA, editors. Philadelphia: Lippincott Williams and Wilkins;2016. p. 322–50.
97. Görlinger K, Sakai T, Dirkmann D, Planinsic RM, Saner FH. Bleeding related to liver transplant. In: Management of Bleeding Patients. Teruya J, editor. Basel: Springer Nature Switzerland AG;2016. p. 263–80.
98. Smith NK, Kim S, Hill B, Goldberg A, DeMaria S, Zerillo J. Transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) in liver transplantation: a case report and focused review. Semin Cardiothorac Vasc Anesth. 2018; 22:180–90.

99. Pandey CK, Singh A, Kajal K, Dhankhar M, Tandon M, Pandey VK, et al. Intraoperative blood loss in orthotopic liver transplantation: The predictive factors. World J Gastrointest Surg. 2015; 7:86–93.

100. Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2013; 19:6919–27.
101. Görlinger K. Coagulation management during liver transplantation. Hamostaseologie. 2006; 26(3 Suppl 1):S64–76.

102. Poon KS, Chen CC, Thorat A, Chiang YY, Jeng LB, Yang HR, et al. Fibrinolysis after reperfusion of liver graft. Acta Anaesthesiol Taiwan. 2015; 53:41–3.

103. Shimauchi T, Yamaura K, Higashi M, Abe K, Yoshizumi T, Hoka S. Fibrinolysis in living donor liver transplantation recipients evaluated using thromboelastometry: impact on mortality. Transplant Proc. 2017; 49:2117–21.

104. Schofield N, Sugavanam A, Thompson K, Mallett SV. No increase in blood transfusions during liver transplantation since the withdrawal of aprotinin. Liver Transpl. 2014; 20:584–90.

105. Dirkmann D, Görlinger K, Peters J. Assessment of early thromboelastometric variables from extrinsically activated assays with and without aprotinin for rapid detection of fibrinolysis. Anesth Analg. 2014; 119:533–42.

106. Kim EH, Song SH, Kim GS, Ko JS, Gwak MS, Lee SK. Evaluation of “flat-line” thromboelastography after reperfusion during liver transplantation. Transplant Proc. 2015; 47:457–9.

107. Abuelkasem E, Lu S, Tanaka K, Planinsic R, Sakai T. Comparison between thrombelastography and thromboelastometry in hyperfibrinolysis detection during adult liver transplantation. Br J Anaesth. 2016; 116:507–12.

108. Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg. 2005; 100:1781–5.

109. Sabate A, Blasi A, Costa M, Reyes R, Beltran J, Torres F. Assessment of rotational thromboelastometry for the prediction of red blood cell requirements in orthotopic liver transplantation. Minerva Anestesiol. 2018; 84:447–54.

110. Caldwell SH, Sanyal AJ. Coagulation disorders and bleeding in liver disease: future directions. Clin Liver Dis. 2009; 13:155–7.

111. Bedreli S, Sowa JP, Malek S, Blomeyer S, Katsounas A, Gerken G, et al. Rotational thromboelastometry can detect factor XIII deficiency and bleeding diathesis in patients with cirrhosis. Liver Int. 2017; 37:562–8.

112. Raspé C, Besch M, Charitos EI, Flöther L, Bucher M, Rückert F, et al. Rotational thromboelastometry for assessing bleeding complications and factor XIII deficiency in cardiac surgery patients. Clin Appl Thromb Hemost. 2018; Advance Access published on Sep 9, 2018, doi:10.1177/1076029618797472.

113. Fenger-Eriksen C, Moore GW, Rangarajan S, Ingerslev J, Sørensen B. Fibrinogen estimates are influenced by methods of measurement and hemodilution with colloid plasma expanders. Transfusion. 2010; 50:2571–6.

114. Noval-Padillo JA, León-Justel A, Mellado-Miras P, Porras-Lopez F, Villegas-Duque D, Gomez-Bravo MA, et al. Introduction of fibrinogen in the treatment of hemostatic disorders during orthotopic liver transplantation: implications in the use of allogenic blood. Transplant Proc. 2010; 42:2973–4.

115. Alamo JM, León A, Mellado P, Bernal C, Marín LM, Cepeda C, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc. 2013; 45:3637–9.
116. Kirchner C, Dirkmann D, Treckmann JW, Paul A, Hartmann M, Saner FH, et al. Coagulation management with factor concentrates in liver transplantation: a single-center experience. Transfusion. 2014; 54:2760–8.

117. Leon-Justel A, Noval-Padillo JA, Alvarez-Rios AI, Mellado P, Gomez-Bravo MA, Álamo JM, et al. Point-of-care haemostasis monitoring during liver transplantation reduces transfusion requirements and improves patient outcome. Clin Chim Acta. 2015; 446:277–83.

118. Zamper RP, Amorim TC, Queiroz VN, Lira JD, Costa LG, Takaoka F, et al. Association between viscoelastic tests-guided therapy with synthetic factor concentrates and allogenic blood transfusion in liver transplantation: a before-after study. BMC Anesthesiol. 2018; 18:198.

119. Sabate A, Gutierrez R, Beltran J, Mellado P, Blasi A, Acosta F, et al. Impact of preemptive fibrinogen concentrate on transfusion requirements in liver transplantation: a multicenter, randomized, double-blind, placebo-controlled trial. Am J Transplant. 2016; 16:2421–9.

120. Fayed NA, Abdallah AR, Khalil MK, Marwan IK. Therapeutic rather than prophylactic platelet transfusion policy for severe thrombocytopenia during liver transplantation. Platelets. 2014; 25:576–86.

121. Debernardi Venon W, Ponzo P, Sacco M, Mengozzi G, Raso S, Valpreda A, et al. Usefulness of thromboelastometry in predicting the risk of bleeding in cirrhotics who undergo invasive procedures. Eur J Gastroenterol Hepatol. 2015; 27:1313–9.

122. Basili S, Raparelli V, Napoleone L, Talerico G, Corazza GR, Perticone F, et al. Platelet count does not predict bleeding in cirrhotic patients: results from the pro-liver study. Am J Gastroenterol. 2018; 113:368–75.

123. Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009; 108:1083–91.

124. Tripodi A, Primignani M, Chantarangkul V, Viscardi Y, Dell’Era A, Fabris FM, et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009; 124:132–6.

125. Mallett SV, Sugavanam A, Krzanicki DA, Patel S, Broomhead RH, Davidson BR, et al. Alterations in coagulation following major liver resection. Anaesthesia. 2016; 71:657–68.

126. Saner FH, Kirchner C. Monitoring and treatment of coagulation disorders in end-stage liver disease. Visc Med. 2016; 32:241–8.

127. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017; 112:274–81.

128. Abuelkasem E, Mazzeffi MA, Lu SY, Planinsic RM, Sakai T, Tanaka KA. Ex vivo evaluation of 4 different viscoelastic assays for detecting moderate to severe coagulopathy during liver transplantation. Liver Transpl. 2016; 22:468–75.

129. Bedreli S, Sowa JP, Gerken G, Saner FH, Canbay A. Management of acute-on-chronic liver failure: rotational thromboelastometry may reduce substitution of coagulation factors in liver cirrhosis. Gut. 2016; 65:357–8.

130. Abuelkasem E, Hasan S, Mazzeffi MA, Planinsic RM, Sakai T, Tanaka KA. Reduced requirement for prothrombin complex concentrate for the restoration of thrombin generation in plasma from liver transplantrecipients. Anesth Analg. 2017; 125:609–15.
131. Lodge JP, Jonas S, Jones RM, Olausson M, Mir-Pallardo J, Soefelt S, et al. Efficacy and safety of repeated perioperative doses of recombinant factor VIIa in liver transplantation. Liver Transpl. 2005; 11:973–9.

132. Simpson E, Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2012; (3):CD005011.

133. Kettner SC, Gonano C, Seebach F, Sitzwohl C, Acimovic S, Stark J, et al. Endogenous heparin-like substances significantly impair coagulation in patients undergoing orthotopic liver transplantation. Anesth Analg. 1998; 86:691–5.

134. Senzolo M, Agarwal S, Zappoli P, Vibhakorn S, Mallett S, Burroughs AK. Heparin-like effect contributes to the coagulopathy in patients with acute liver failure undergoing liver transplantation. Liver Int. 2009; 29:754–9.

135. Yassen K, Refaat E, Helal S, Metwally A, Youssef S, Görlinger K. Perioperative heparinase rotational thromboelastometry monitoring during and after adult living related liver transplantation. Eur J Anaesthesiol. 2018; 35(e-Suppl 56):286.
136. Gouvêa G, Toledo R, Diaz R, Auler L, Enne M, Martinho JM. Protamine sulphate for treatment of severe post-reperfusion coagulopathy in pediatric liver transplantation. Pediatr Transplant. 2009; 13:1053–7.

137. Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014; 18:549.

138. Rossetto V, Spiezia L, Senzolo M, Rodriguez-Castro KI, Maggiolo S, Simioni P. Whole blood rotation thromboelastometry (ROTEMⓇ) profiles in subjects with non-neoplastic portal vein thrombosis. Thromb Res. 2013; 132:e131–4.

139. Zanetto A, Senzolo M, Vitale A, Cillo U, Radu C, Sartorello F, et al. Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma. Dig Liver Dis. 2017; 49:440–5.

140. Blasi A, Molina V, Sanchez-Cabús S, Balust J, Garcia-Valdecasas JC, Taura P. Prediction of thromboembolic complications after liver resection for cholangiocarcinoma: is there a place for thromboelastometry? Blood Coagul Fibrinolysis. 2018; 29:61–6.
141. Kamel Y, Hassanin A, Ahmed AR, Gad E, Afifi M, Khalil M, et al. Perioperative thromboelastometry for adult living donor liver transplant recipients with a tendency to hypercoagulability: a prospective observational cohort study. Transfus Med Hemother. 2018; 45:404–12.

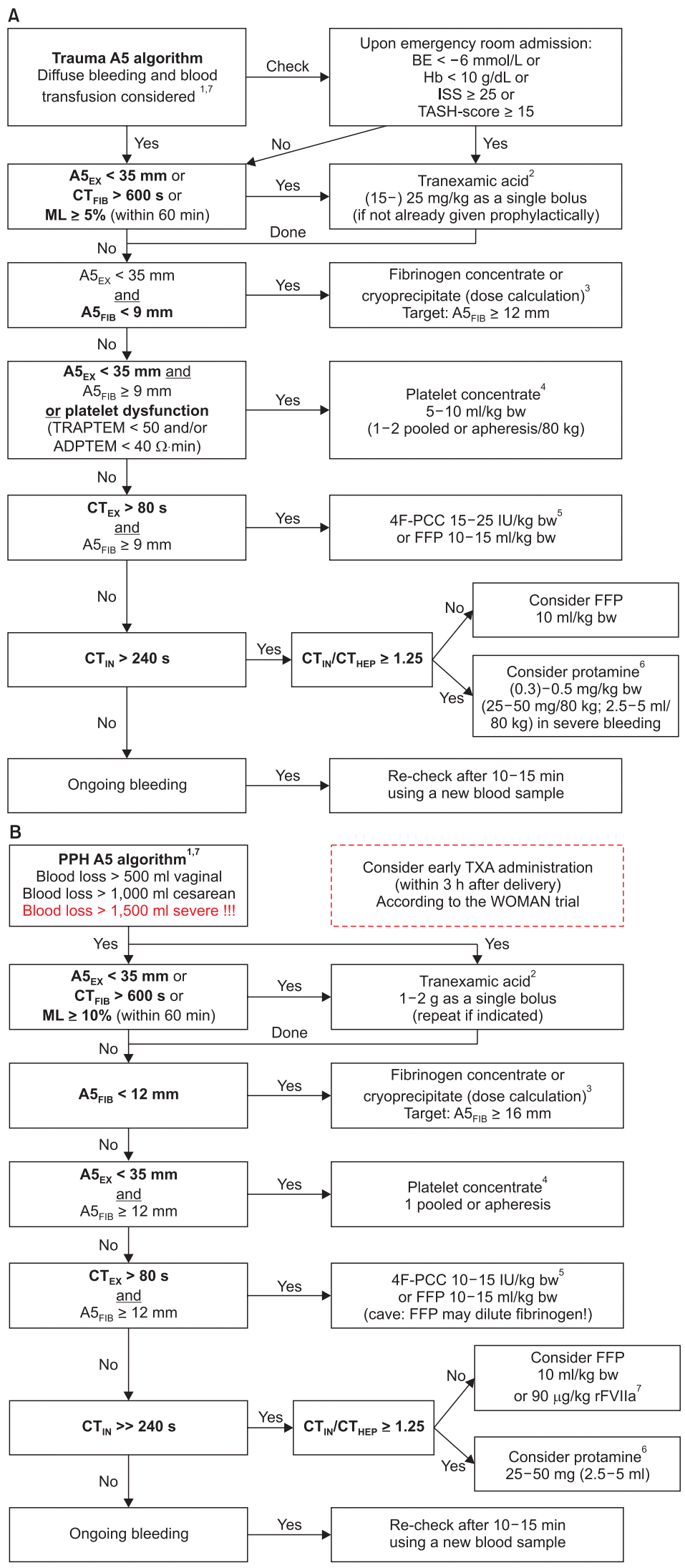
142. CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010; 376:23–32.
143. CRASH-2 collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011; 377:1096–101.
144. Gayet-Ageron A, Prieto-Merino D, Ker K, Shakur H, Ageron FX, Roberts I. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018; 391:125–32.
145. Görlinger K, Dirkmann D, Hanke AA. Rotational thromboelastometry (ROTEM). In: Trauma Induced Coagulopathy. Gonzalez E, Moore HB, Moore EE, editors. Basel: Springer Nature Switzerland AG;2016. p. 267–98.
146. Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013; 75:961–7.

147. Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, Silliman CC. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016; 56 Suppl 2:S110–4.

148. Roberts I. Fibrinolytic shutdown: fascinating theory but randomized controlled trial data are needed. Transfusion. 2016; 56 Suppl 2:S115–8.

149. Maegele M. Uncritical use of tranexamic acid in trauma patients : Do no further harm! Unfallchirurg. 2016; 119:967–72.
150. Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, Livingstone AS, et al. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014; 76:1373–8.

151. Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, et al. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg. 2015; 78:905–9.

152. Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg. 2017; 224:575–82.
153. Moore HB, Moore EE, Huebner BR, Stettler GR, Nunns GR, Einersen PM, et al. Tranexamic acid is associated with increased mortality in patients with physiological fibrinolysis. J Surg Res. 2017; 220:438–43.

154. Meizoso JP, Dudaryk R, Mulder MB, Ray JJ, Karcutskie CA, Eidelson SA, et al. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J Trauma Acute Care Surg. 2018; 84:426–32.

155. Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of military use of tranexamic acid and associated thromboembolicevents. JAMA Surg. 2018; 153:169–75.
156. Stettler GR, Moore EE, Moore HB, Nunns GR, Silliman CC, Banerjee A, et al. Redefining postinjury fibrinolysis phenotypes using two viscoelastic assays. J Trauma Acute Care Surg. 2019; 86:679–85.

157. Harr JN, Moore EE, Chin TL, Chapman MP, Ghasabyan A, Stringham JR, et al. Viscoelastic hemostatic fibrinogen assays detect fibrinolysis early. Eur J Trauma Emerg Surg. 2015; 41:49–56.

158. Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Curr Opin Anaesthesiol. 2012; 25:229–34.

159. Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEMⓇ) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016; 24:114.

160. Davenport RA, Brohi K. Cause of trauma-induced coagulopathy. Curr Opin Anaesthesiol. 2016; 29:212–9.

161. Wanderer JP, Nathan N. Massive transfusion protocols: when to turn on, and off, the fire hose. Anesth Analg. 2017; 125:1827.
162. Foster JC, Sappenfield JW, Smith RS, Kiley SP. Initiation and termination of massive transfusion protocols: current strategies and future prospects. Anesth Analg. 2017; 125:2045–55.
163. Hagemo JS, Stanworth S, Juffermans NP, Brohi K, Cohen M, Johansson PI, et al. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: a multicentre observational study. Crit Care. 2014; 18:R52.

164. Inaba K, Rizoli S, Veigas PV, Callum J, Davenport R, Hess J, et al. 2014 Consensus conference on viscoelastic test-based transfusion guidelines for early trauma resuscitation: report of the panel. J Trauma Acute Care Surg. 2015; 78:1220–9.
165. Maegele M, Schöchl H, Menovsky T, Maréchal H, Marklund N, Buki A, et al. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017; 16:630–47.

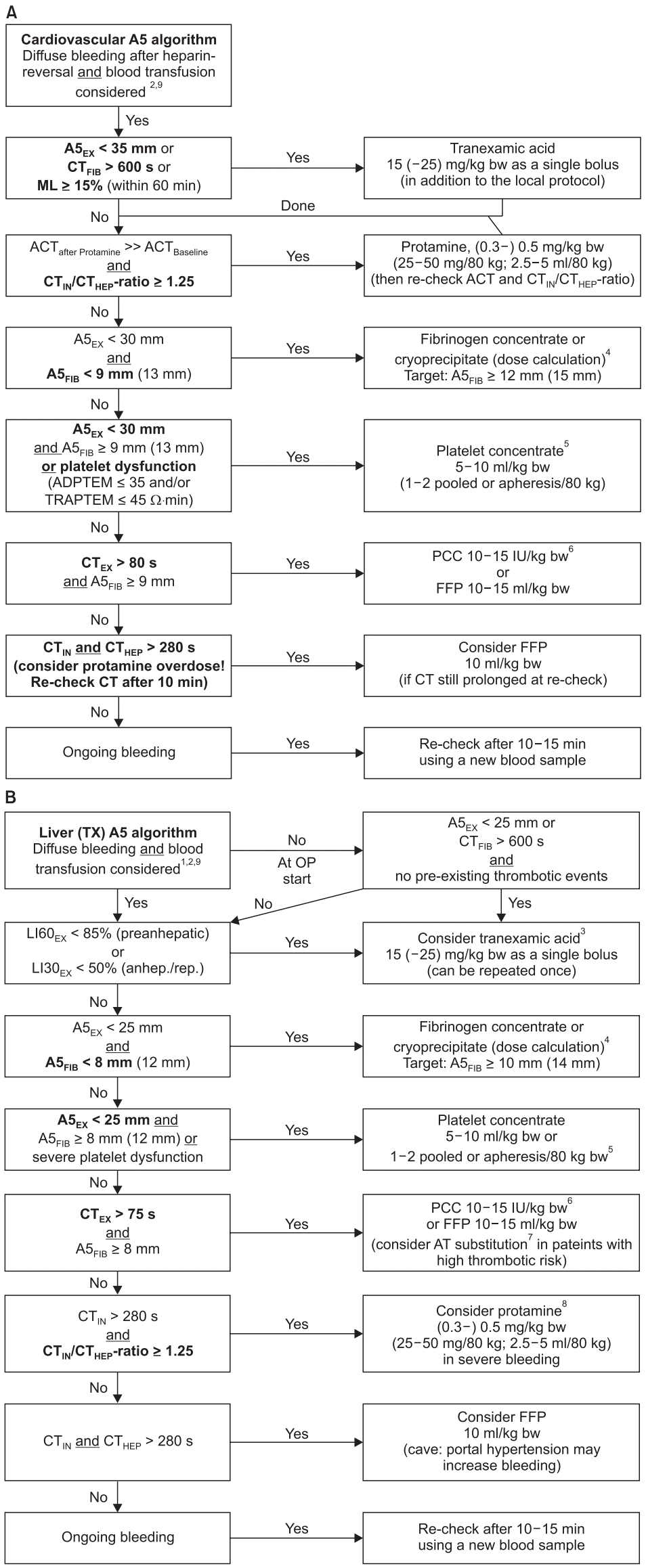
167. Baksaas-Aasen K, Van Dieren S, Balvers K, Juffermans NP, Næss PA, Rourke C, et al. Data-driven development of ROTEM and TEG algorithms for the management of trauma emorrhage: a prospective observational multicenter study. Ann Surg. 2018; Advance Access published on May 23, 2018, doi:10.1097/SLA.0000000000002825.
168. Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe-Meyer N, Voelckel W, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thromb Haemost. 2011; 106:322–30.
169. Chapman MP, Moore EE, Moore HB, Gonzalez E, Morton AP, Silliman CC, et al. Early TRAP pathway platelet inhibition predicts coagulopathic hemorrhage in trauma. Shock. 2015; 43(Suppl 1):33.
170. Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg. 2017; 83:388–97.

171. Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007; 62:112–9.

172. Innerhofer P, Fries D, Mittermayr M, Innerhofer N, von Langen D, Hell T, et al. Reversal of trauma-induced coagulopathy using firstline coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017; 4:e258–71.

173. Grottke O, Rossaint R. Coagulation factor concentrates and point-of-care coagulation monitoring: both might be essential for optimal treatment of trauma-induced coagulopathy. Lancet Haematol. 2017; 4:e246–7.

174. Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016; 20:100.

175. Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012; 73:60–6.

176. Holley AD, Reade MC. The ‘procoagulopathy’ of trauma: too much, too late? Curr Opin Crit Care. 2013; 19:578–86.
177. Moore HB, Moore EE, Liras IN, Wade C, Huebner BR, Burlew CC, et al. Targeting resuscitation to normalization of coagulating status: Hyper and hypocoagulability after severe injury are both associated with increased mortality. Am J Surg. 2017; 214:1041–5.

178. Dhillon NK, Smith EJ, Ko A, Harada MY, Yang AR, Patel KA, et al. The risk factors of venous thromboembolism in massively transfused patients. J Surg Res. 2018; 222:115–21.

179. Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012; 109:851–63.

180. Collins P, Abdul-Kadir R, Thachil J. Management of coagulopathy associated with postpartum hemorrhage: guidance from the SSC of the ISTH. J Thromb Haemost. 2016; 14:205–10.

181. McNamara H, Mallaiah S, Barclay P, Chevannes C, Bhalla A. Coagulopathy and placental abruption: changing management with ROTEMguided fibrinogen concentrate therapy. Int J Obstet Anesth. 2015; 24:174–9.

182. James AH, McLintock C, Lockhart E. Postpartum hemorrhage: when uterotonics and sutures fail. Am J Hematol. 2012; 87 Suppl 1:S16–22.

183. Lockhart E. Postpartum hemorrhage: a continuing challenge. Hematology Am Soc Hematol Educ Program. 2015; 2015:132–7.

184. Kaufner L, Henkelmann A, von Heymann C, Feldheiser A, Mickley L, Niepraschk-von Dollen K, et al. Can prepartum thromboelastometryderived parameters and fibrinogen levels really predict postpartum hemorrhage? J Perinat Med. 2017; 45:427–35.

185. Mallaiah S, Barclay P, Harrod I, Chevannes C, Bhalla A. Introduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in major obstetric haemorrhage. Anaesthesia. 2015; 70:166–75.

186. Snegovskikh D, Souza D, Walton Z, Dai F, Rachler R, Garay A, et al. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018; 44:50–6.

187. Girard T, Mörtl M, Schlembach D. New approaches to obstetric hemorrhage: the postpartum hemorrhage consensus algorithm. Curr Opin Anaesthesiol. 2014; 27:267–74.
188. Butwick AJ, Goodnough LT. Transfusion and coagulation management in major obstetric hemorrhage. Curr Opin Anaesthesiol. 2015; 28:275–84.

189. Collins PW, Bell SF, de Lloyd L, Collis RE. Management of postpartum haemorrhage: from research into practice, a narrative review of the literature and the Cardiff experience. Int J Obstet Anesth. 2019; 37:106–17.

190. Hanke AA, Elsner O, Görlinger K. Spinal anaesthesia and caesarean section in a patient with hypofibrinogenaemia and factor XIII deficiency. Anaesthesia. 2010; 65:641–5.

191. Truong HT, Browning RM. Anaphylaxis-induced hyperfibrinolysis in pregnancy. Int J Obstet Anesth. 2015; 24:180–4.

192. Annecke T, Geisenberger T, Kürzl R, Penning R, Heindl B. Algorithm-based coagulation management of catastrophic amniotic fluid embolism. Blood Coagul Fibrinolysis. 2010; 21:95–100.

193. Collins NF, Bloor M, McDonnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anesth. 2013; 22:71–6.

194. Chen CH, Lee KC, Hsieh YJ. Amniotic fluid embolism complicated with pulmonary embolism during cesarean section: management with TEE and ROTEMⓇ. Asian J Anesthesiol. 2017; 55:93–4.
195. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389:2105–16.
196. Roberts I, Shakur H, Fawole B, Kuti M, Olayemi O, Bello A, et al. Haematological and fibrinolytic status of Nigerian women with postpartum haemorrhage. BMC Pregnancy Childbirth. 2018; 18:143.

197. Dallaku K, Shakur H, Edwards P, Beaumont D, Roberts I, Huque S, et al. Statistical analysis plan for the WOMAN-ETAPlaT study: effect of tranexamic acid on platelet function and thrombin generation. Wellcome Open Res. 2016; 1:30.

198. Charbit B, Mandelbrot L, Samain E, Baron G, Haddaoui B, Keita H, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007; 5:266–73.

199. Mallaiah S, Chevannes C, McNamara H, Barclay P. A reply. Anaesthesia. 2015; 70:760–1.

200. Smith RA, Mallaiah S, Chevannes C, McNamara H. Lessons from four years’ experience in the use of ROTEM-guided fibrinogen concentrate in major obstetric haemorrhage. Int J Obstet Anesth. 2017; 31(Suppl 1):S7.
201. Smith RA, Mallaiah S, Barclay P, Chevannes C, McNamara H. Improved outcomes with ROTEM-guided fibrinogen concentrate in major obstetric haemorrhage. Int J Obstet Anesth. 2017; 31(Suppl 1):S14.
202. Wikkelsø AJ, Edwards HM, Afshari A, Stensballe J, Langhoff-Roos J, Albrechtsen C, et al. Pre-emptive treatment with fibrinogen concentrate for postpartum haemorrhage: randomized controlled trial. Br J Anaesth. 2015; 114:623–33.
203. Collins PW, Cannings-John R, Bruynseels D, Mallaiah S, Dick J, Elton C, et al. Viscoelastometric-guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double-blind randomized controlled trial. Br J Anaesth. 2017; 119:411–21.

204. Collins PW, Cannings-John R, Bruynseels D, Mallaiah S, Dick J, Elton C, et al. Viscoelastometry guided fresh frozen plasma infusion for postpartum haemorrhage: OBS2, an observational study. Br J Anaesth. 2017; 119:422–34.

205. American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015; 122:241–75.
206. Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R, et al. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia. 2016; 71:829–42.

207. Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017; 34:332–95.
208. Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guidelin. Br J Haematol. 2018; 182:789–806.
209. Schlembach D, Helmer H, Henrich W, von Heymann C, Kainer F, Korte W, et al. Peripartum Haemorrhage, Diagnosis and Therapy. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No.015/063, March 2016). Geburtshilfe Frauenheilkd. 2018; 78:382–99.

210. Roullet S, de Maistre E, Ickx B, Blais N, Susen S, Faraoni D, et al. Position of the French working group on perioperative haemostasis (GIHP) on viscoelastic tests: what role for which indication in bleeding situations? Anaesth Crit Care Pain Med. 2018. Advance Access published on Feb 3, 2018, doi:10.1016/j.accpm.2017.12.014.

211. Collis R. Coagulation point-of-care testing on the labour ward should be mandatory. Int J Obstet Anesth. 2016; 27:66–9.

212. Abir G, Mhyre J. Maternal mortality and the role of the obstetric anesthesiologist. Best Pract Res Clin Anaesthesiol. 2017; 31:91–105.

213. McDonnell NJ, Browning R. How to replace fibrinogen in postpartum haemorrhage situations? (Hint: Don’t use FFP!). Int J Obstet Anesth. 2018; 33:4–7.

214. Pearse BL, Smith I, Faulke D, Wall D, Fraser JF, Ryan EG, et al. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang. 2015; 109:267–79.

215. Trevisan D1, Zavatti L, Gabbieri D, Pedulli M, Giordano G, Meli M. Point-of-care-based protocol with first-line therapy with coagulation factor concentrates is associated with decrease allogenic blood transfusion and costs in cardiovascular surgery: an Italian single-center experience. Minerva Anestesiol. 2016; 82:1077–88.
216. Vasques F, Spiezia L, Manfrini A, Tarzia V, Fichera D, Simioni P, et al. Thromboelastometry guided fibrinogen replacement therapy in cardiac surgery: a retrospective observational study. J Anesth. 2017; 31:286–90.

217. Mehaffey JH, Schubert SA, Gelvin MG, Charles EJ, Hawkins RB, Johnston LE, et al. A new intraoperative protocol for reducing perioperative transfusionsin cardiac surgery. Ann Thorac Surg. 2017; 104:176–81.
218. Smith I, Pearse BL, Faulke DJ, Naidoo R, Nicotra L, Hopkins P, et al. Targeted bleeding management reduces the requirements for bloodcomponent therapy in lung transplant recipients. J Cardiothorac Vasc Anesth. 2017; 31:426–33.
219. Buscher H, Zhang D, Nair P. A pilot, randomised controlled trial of a rotational thromboelastometry-based algorithm to treat bleeding episodes in extracorporeal life support: the TEM Protocol in ECLS Study (TEMPEST). Crit Care Resusc. 2017; 19(Suppl 1):29–36.
220. Smart L, Mumtaz K, Scharpf D, Gray NO, Traetow D, Black S, et al. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol. 2017; 16:916–23.

221. Schaden E, Kimberger O, Kraincuk P, Baron DM, Metnitz PG, Kozek-Langenecker S. Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. Br J Anaesth. 2012; 109:376–81.

222. Nardi G, Agostini V, Rondinelli B, Russo E, Bastianini B, Bini G, et al. Trauma-induced coagulopathy: impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care. 2015; 19:83.

223. Haas T, Spielmann N, Restin T, Seifert B, Henze G, Obwegeser J, et al. Higher fibrinogen concentrations for reduction of transfusion requirements during major paediatric surgery: A prospective randomised controlled trial. Br J Anaesth. 2015; 115:234–43.
224. Guan J, Cole CD, Schmidt MH, Dailey AT. Utility of intraoperative rotational thromboelastometry in thoracolumbar deformity surgery. J Neurosurg Spine. 2017; 27:528–33.

225. Naik BI, Pajewski TN, Bogdonoff DI, Zuo Z, Clark P, Terkawi AS, et al. Rotational thromboelastometry-guided blood product management in major spine surgery. J Neurosurg Spine. 2015; 23:239–49.

226. Prat NJ, Meyer AD, Ingalls NK, Trichereau J, DuBose JJ, Cap AP. Rotational thromboelastometry significantly optimizes transfusion practices for damage control resuscitation in combat casualties. J Trauma Acute Care Surg. 2017; 83:373–80.

227. Fries D, Innerhofer P, Spahn DR. Transfusion approaches and mortality in trauma patients: a narrativereview. Semin Thromb Hemost. 2017; 43:759–71.
228. Stein P, Kaserer A, Sprengel K, Wanner GA, Seifert B, Theusinger OM, et al. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia. 2017; 72:1317–26.

229. Meybohm P, Herrmann E, Steinbicker AU, Wittmann M, Gruenewald M, Fischer D, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg. 2016; 264:203–11.
230. Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017; 57:1347–58.

231. Leahy MF, Roberts H, Mukhtar SA, Farmer S, Tovey J, Jewlachow V, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014; 54:1133–45.

232. Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Füllenbach C, et al. Multimodal patient blood management program based on a three-pillarstrategy: a systematic review and meta-analysis. Ann Surg. 2019; 269:794–804.
233. Pagano D, Milojevic M, Meesters MI, Benedetto U, Bolliger D, von Heymann C, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018; 53:79–111.

235. National Blood Authority Australia. National blood management implementation strategy 2017-2021. Better management of patients’ blood … better patient outcomes. 2017. Available from
https://www.blood.gov.au/implementing-pbm.
238. Görlinger K, Kozek-Langenecker SA. Economic aspects and organization. In: Perioperative Hemostasis. Coagulation for Anesthesiologists. Marcucci CE, Schoettker P, editors. Basel: Springer Publishing;2015. p. 421–45.
239. Fuller RL, McCullough EC, Bao MZ, Averill RF. Estimating the costs of potentially preventable hospital acquired complications. Health Care Financ Rev. 2009; 30:17–32.
240. Görlinger K, Dirkmann D, Tanaka AK, Shander A, Spahn DR, Hofmann A. Implementation of thromboelastometry-guided patient blood management results in cost-savings for blood product acquisition and potentially preventable hospital acquired complications. In : 10th Annual Meeting of the German Society of Health Economics (DGGÖ); 2018. p. 228–9.
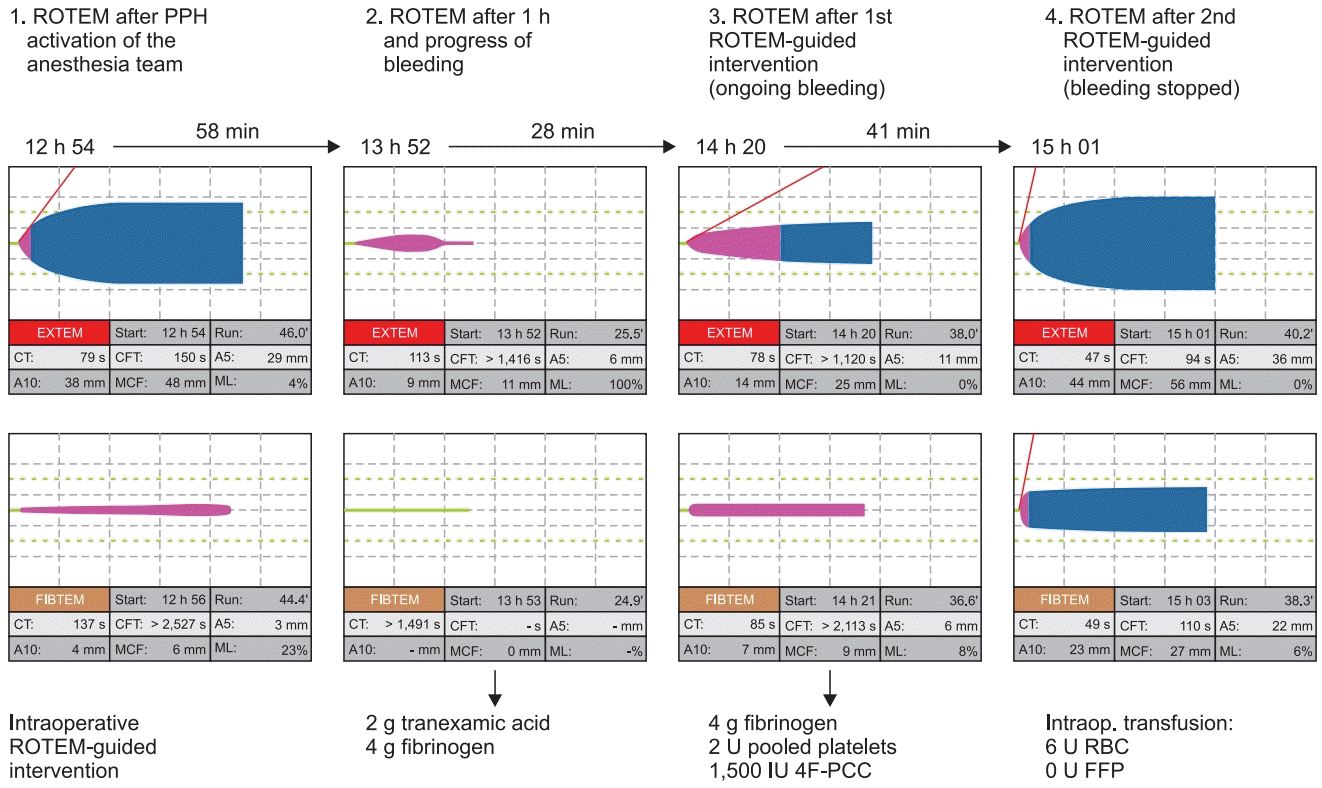




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download