Abstract
High-flow nasal oxygenation (HFNO) is a promising new technique for anesthesiologists. The use of HFNO during the induction of anesthesia and during upper airway surgeries has been initiated, and its applications have been rapidly growing ever since. The advantages of this technique include its easy set-up, high tolerability, and its abilities to produce positive airway pressure and a high fraction of inspired oxygen and to influence the clearance of carbon dioxide to some extent. HFNO, via a nasal cannula, can provide oxygen both to patients who can breathe spontaneously and to those who are apneic; further, this technique does not interfere with bag-mask ventilation, attempts at laryngoscopy for tracheal intubation, and surgical procedures conducted in the airway. In this review, we describe the techniques associated with HFNO and the advantages and disadvantages of HFNO based on the current state of knowledge.
Airway management is an essential skill that all anesthesiologists require. As the current airway management methods are still not optimal, anesthesiologists need to be continuously updated with new developments related to airway devices and techniques [1]. Among these developments, high-flow nasal oxygenation (HFNO) is particularly prominent. Although the use of this technique is spreading globally, there are some controversies regarding its indications and the advantages of having the required skills to use it. Therefore, in this narrative review, we focus on the new emerging airway management skill of performing HFNO, and we describe the potential role of HFNO based on the current state of knowledge.
HFNO is a method that provides oxygen at a high flow rate of over 15 L/min (which is the maximum flow rate for a conventional nasal cannula) through a patient’s nasal opening [2]. The device is able to titrate the fractional inspired oxygen (FiO2) to up to 1.0 and to consistently deliver a highly oxygenated flow to the alveoli because of its ability to provide higher flow rates than that of the usual inspiratory flow and to reduce the entrainment of room air [2]. HFNO generates a low level of continuous positive airway pressure of 2.7–7.4 cmH2O, facilitates the washout of the nasopharyngeal dead space, reduces the nasopharyngeal resistance, increases alveolar recruitment, decreases the work of breathing, and prevents the development of atelectasis and bronchospasm [2–4]. Specially designed equipment is required for HFNO because the oxygen flow should be at adequately high temperatures and humidity levels, which prevents the feeling of dryness in the nasal cavity of patients and promotes tolerance for the high-flow rate [3]. Additionally, humidified and heated inspired oxygen lessens the energy cost involved in gas conditioning [5], prevents mucociliary damage, and facilitates mucociliary clearance [3,6].
HFNO has proven highly effective for patients admitted to the intensive care unit (ICU) [7] or to the post-anesthetic care unit after surgery [3]. It is evident that HFNO enhances oxygenation in patients who develop hypoxemic acute respiratory failure and that it helps to avert the need for reintubation after extubation in the ICU [3]. A meta-analysis reported that HFNO, when employed postoperatively, reduced the length of hospital stay for adult patients [8]. Following this meta-analysis report, another study confirmed that postoperative HFNO reduced the length of hospital stay and the incidence of re-admission to the ICU in the case of cardiac surgical patients with pre-existing respiratory diseases [7].
The reported side effects from the trials were minor, such as being sensitive to noise, feeling hot, and developing a runny nose [3]. The nasal interface of the HFNO system is composed of soft silicone with a wider bore compared to that of traditional nasal prongs. HFNO is generally well tolerated compared to other means of oxygen supplementation, such as a low-flow nasal cannula or a facemask [9]; further, patients can tolerate HFNO with a flow rate of up to 100 L/min without experiencing any discomfort, and some may even fall asleep [4].
HFNO has potential contraindications, including in cases involving severe nasal obstruction, copious nasal bleeding, recent nasal trauma, recent nasal surgery, significantly raised intracranial pressure, and base-of-skull fractures [10].
Recently, HFNO has been applied during anesthesia management, including during the anesthesia induction period and intraoperative period (Fig. 1). For this purpose, a system with different characteristics (OptiflowTM; Fisher & Paykel Healthcare, New Zealand) has been developed. Optiflow consists of a flow meter, humidifier, heating system, heated non-condensing circuit, nasal cannula, head strap, and an oxygen connector for gas supply (Fig. 2). It is different from the Airvo system (AirvoTM system, Fisher & Paykel Healthcare Ltd., New Zealand) in its use in the ICU as the FiO2 is fixed at 1.0 in order to facilitate the installation of the device within 2–3 min [11]. The oxygen flow rate can be increased to up to 70 L/min, which is more than that of Airvo (2–60 L/min). Due to the FiO2 of 1.0 and its higher flow rate, Optiflow is mostly indicated for adults and children who weigh more than 10 kg. Airvo is more appropriate than Optiflow for children who weigh less than 10 kg due to the proper titration of FiO2 to less than 1.0 (range: 21–100%) and the flow rate of less than 20 L/min. The heating and active humidifying of oxygen flow performed in Optiflow are similar to those in Airvo (37℃ and 44 mgH2O/L). It is recommended that this equipment be turned on approximately 5 min before its use to ensure adequate humidification and heating [10].
When general anesthesia is induced and a neuromuscular blocking agent is administered, the risk of hypoxemia arises after the induction of anesthesia if the facemask ventilation is inadequate and if tracheal intubation is difficult or has failed. In the case of difficult oxygenation during the induction of anesthesia, a high risk of severe complications, such as hemodynamic instability, dysrhythmias, hypoxic brain injury, cardiac arrest, and even death, develops [12]. One effective method to minimize hypoxemia-related complications during the induction of anesthesia is adequate oxygenation.
The oxygenation process during the induction of anesthesia can be divided into 2 phases based on the time points at which they are performed: preoxygenation and peroxygenation. Preoxygenation is defined as the administration of oxygen before the induction of anesthesia, and its purpose is to maximize the amount of a ‘safe’ apnea time (apnea time without hypoxemia) after the induction of anesthesia and to delay the onset of hypoxemia if airway management is unexpectedly difficult [12,13]. Peroxygenation is the oxygenation performed from the time point at which anesthesia is induced until when the airway is completely secured. After the induction of anesthesia, a patient’s spontaneous breathing can either be maintained or stopped. In the latter case, the usual method is the application of intermittent positive pressure ventilation via a facemask. If this is not possible, an alternative method is the insufflation of oxygen into the pharyngeal cavity, and this method is called ‘apneic oxygenation.’
For adults, the conventional method of preoxygenation is to provide oxygen using an FiO2 of 1.0 with a flow rate of 10–12 L/min through a tightly fitting facemask placed over a patient’s nose and mouth [14,15]. The duration of preoxygenation should be titrated to denitrogenate the expiratory reserve and residual lung volume [16]. It is recommended to then confirm adequate preoxygenation via an end-tidal oxygen partial pressure (EtO2) that exceeds 90%. In healthy adults, this endpoint can be achieved within 3–5 min when using tidal volume breathing through a facemask [17].
During preoxygenation, the method via which the patient breathes is crucial, and this affects the time needed for adequate oxygenation [16]. The 2 most representative methods are tidal volume breathing for 3 min and vital capacity breathing for 8 breaths within 60 s [18]. Adequate preoxygenation enables a healthy adult to endure apnea for approximately 7–10 min without developing hypoxemia [18].
Apneic oxygenation can be employed to further delay the onset of desaturation. Employing apneic oxygenation can result in additional time to consider and attempt alternative airway management options when the airway cannot easily be secured. While the patient is in this less stressful condition, it is critical for the physician to focus on preventing an emergency situation and on making the right decision for the patient’s safety.
The concept of apneic oxygenation was established in 1908.1) The underlying physiology involves the passive movement of oxygen from the nasopharynx or the oropharynx to the alveoli; consequently, oxygen is taken up into the bloodstream even without active lung expansion [19,20]. This gas movement occurs because oxygen and carbon dioxide differ in the flow rates associated with their absorption and excretion between the alveoli and bloodstream (O2: 250 ml/min and CO2: 8–20 ml/min). This creates a sub-atmospheric pressure in the alveoli and a pressure gradient between the upper airway and the alveoli. Owing to this, oxygen is drawn from the pharynx into the alveoli. Therefore, if oxygenation were insufflated through the nasal or oral (buccal) route, it would prevent rapid desaturation even when a patient is apneic [21]. There are several available devices, such as nasal prongs, nasopharyngeal catheters, oropharyngeal catheters, an adapted Ring-Adair-Elwin (RAE) tube, and a modified laryngoscope, that currently exist for this purpose.
Apneic oxygenation through a nasal catheter or via prongs, with an oxygen flow rate of 3–10 L/min, is generally useful in delaying desaturation in adult patients with physical statuses of American Society of Anesthesiologists (ASA) 1 or 2 [22]. In a retrospective study involving 728 patients [23], the incidence of desaturation (peripheral capillary oxygen saturation [SpO2] < 93%) was decreased from 23% to 17% by the implementation of apneic oxygenation (with an oxygen flow rate of 15 L/min) via a nasal cannula. Likewise, in a study involving 127 patients with intracranial hemorrhage who required rapid-sequence induction of anesthesia in the emergency department [24], nasal prongs with an oxygen flow of 5–15 L/min during apnea reduced the incidence of desaturation (SpO2 < 90%) from 29% to 7%. In obese patients (with a mean body mass index [BMI]: 31.2 kg/m2), nasal prongs with an oxygen flow of 5 L/min extended the safe apnea time (SpO2 ≥ 95%) from 3.5 to 5.3 min [25]. An oxygen flow of 10 L/min via nasal prongs together with an oxygen flow of 15 L/min via a facemask for 3 min increased the EtO2 even in the case of a gas leakage around the facemask [26].
A previous study [27] involving 56 adult patients classified as ASA 1–2 demonstrated that the administration of 5 L/min of oxygen via a nasopharyngeal catheter was a superior method in preventing desaturation compared to providing oxygenation via nasal prongs; the possible reason for this is that a nasopharyngeal catheter delivers oxygen more effectively to the alveoli because of the shorter distance from the oxygen outlet to the laryngeal inlet. The insufflation of oxygen at a flow rate of 10 L/min via a RAE tube with its tip placed into the oral cavity (buccal route) extended the median safe apnea time (tracheal oxygen concentrations > 94%) from 447 to 750 s in adult patients [28]. However, in 1 out of 10 patients, the SpO2 decreased to less than 95% at the 9.5 min time point, and in another patient, the SpO2 fell to less than 50% due to the soft tissues occluding the tip of the RAE tube [28]. A similar study was performed on obese patients with BMIs of 30–40 kg/m2 in which apneic oxygenation with a flow rate of 10 L/min via a RAE tube having an internal diameter of 3.5 mm extended the median safe apnea time (SpO2 ≥ 95%) from 296–750 s [29]. In infants and small children without cardiopulmonary diseases, oxygen insufflation at a rate of 4 L/min through a tube attached to the side channel of a videolaryngoscope extended the safe apnea time (SpO2 ≥ 95%) by an average of 30 s (mean time: from 131 to 166 s) [30].
Apneic oxygenation devices that provide oxygen at a flow rate of less than 15 L/min have several limitations. First, the resulting FiO2 is unpredictable and limited due to the entrainment of room air [21]. Second, the airflow is cold and dry, impairing the mucociliary function and thereby leading to a risk of triggering bronchoconstriction. Third, the tip of a nasopharyngeal catheter or of a RAE tube can be misplaced or occluded by the surrounding tissues, thereby resulting in unexpected desaturation. Fourth, oxygen insufflation via a tube that is attached to the side channel of a laryngoscope can be performed only when the laryngoscope is placed in the patient’s oropharynx. Lastly, when oxygen insufflation is performed while the patient is apneic, the clearance of carbon dioxide from the body cannot be expected [31].
The efficacy of HFNO therapy in extending the safe apnea time before the airway is secured has been examined via several clinical studies, the focus of which could be categorized into the following topics: preoxygenation, induction of general anesthesia, rapid-sequence induction, and awake tracheal intubation (Table 1).
One of the benefits of HFNO is that it can easily and comfortably be applied to a patient who is awake until anesthesia is induced. A study compared HFNO with an oxygen flow of 60 L/min for 3 min with oxygenation via a facemask (with an oxygen flow of 10 L/min) in 10 adult volunteers who were awake [32]. The resulting mean EtO2 was similar between both techniques (86 kPa for HFNO and 89 kPa for a facemask). Nevertheless, in the case of HFNO, the EtO2 decreased to 49 kPa when the volunteers opened their mouths, indicating that HFNO may be effective only with the mouth closed.
In another observational study, HFNO with an oxygen flow of 70 L/min was applied to 21 adult volunteers who were awake, and it was observed that while the volunteers were closing their mouths, the EtO2 increased from 14–17% to 78–92% in 3 min [33]. Nevertheless, in half of the volunteers, the EtO2 did not reach 90%. In addition, 1 patient experienced discomfort, and the test was stopped midway; further, an additional 4 patients reported experiencing moderate discomfort (5 or 6 on a visual analogue scale). Although it may be technically difficult to measure the EtO2 accurately during HFNO, it is also possible that HFNO may not reliably increase the EtO2 during preoxygenation for 3 min. In addition, patients in respiratory distress situations inevitably need to breathe with an open mouth, and it may be difficult for them to follow instructions requiring them to close their mouths [34]. The optimal parameters for the application of HFNO, such as the duration of preoxygenation, breathing pattern, or mouth status, need to be determined. In reported studies, different durations, ranging between 3–5 min, of HFNO were applied before the start of anesthesia induction [10,32,35–37]. To determine the optimal preoxygenation time with HFNO, apart from the EtO2, new criteria, such as the oxygen reserve index sensor, should be considered in future clinical trials.
In obese patients, when preoxygenation is carried out through a facemask, the safe apnea time period is as short as 1–3 min following preoxygenation compared with 7–10 min in healthy adult patients [18,38]. This is due to obesity-related physiological changes such as decreased functional residual capacity, increased oxygen consumption, and increased closing volume [15]. In a study involving 33 morbidly obese patients (BMI ≥ 35 kg/m2) [39], HFNO at a flow rate of 50 L/min with the patient’s mouth closed was superior to facemask oxygenation in achieving a high arterial partial pressure of oxygen (PaO2). After 3 min of preoxygenation with the patient in a 30° head-up position, the median PaO2 was 380 mmHg in the HFNO group and 337 mmHg in the facemask group. None of the patients developed complications due to epistaxis or gastric aspiration when using HFNO. Therefore, in obese patients, the efficacy of HFNO is increased by placing the patient in a head-up position.
In obstetric patients, it is particularly important to make sure that the EtO2 reaches over 90% by employing at least 3 min of tidal volume breathing of 100% oxygen [19,40] because the rate at which difficult intubation occurs in these patients may be 10 times higher than in the general population [12,41]. In addition, the safe apnea time period is relatively short due to several pregnancy-related changes such as airway edema, decreased functional residual capacity, increased oxygen consumption, and a higher risk of aspiration, as well as due to emergency surgery [40].
A computational model that simulates the effects of apneic oxygenation during rapid-sequence induction of anesthesia in obstetric women has indicated that the increase in FiO2 to up to 1.0 extends the safe apnea time [42]. The successful use of HFNO in a pregnant woman with severe cardiopulmonary disease has been reported in the case of a 27-year-old pregnant woman with acute respiratory distress syndrome and heart failure who required an emergency Cesarean section [11]. The patient had dyspnea and exhibited significant mouth breathing, and her SpO2 was 80%. Oxygen at a flow rate of 9 L/min via a facemask increased the SpO2 to 95%, and HFNO at a flow rate of 70 L/min with an FiO2 of 1.0 further increased the SpO2 to 98% within 5 min even though the patient breathed predominantly through her mouth. After the induction of anesthesia, tracheal intubation was successfully performed with the SpO2 maintained at 98%.
In contrast, the results of another study have cast a doubt on the efficacy of HFNO [35] in pregnant women: following HFNO for 3 min (30 L/min for 30 s, followed by 50 L/min for 150 s), an EtO2 of 90% was achieved in merely 44 out of 73 (60%) pregnant women. In addition, although HFNO received similar scores associated with the comfort of its use to those of a facemask, only 56% of women preferred HFNO to the facemask. These results are similar to those associated with non-pregnant patients.
After the induction of anesthesia and a neuromuscular blockade, oxygenation is usually performed by intermittent positive pressure ventilation via a facemask. When a facemask is used, the mask needs to be removed while attempting tracheal intubations, and thus, no oxygen is supplied during these attempts. If the time taken for tracheal intubation is prolonged and if difficulties, such as in advancing the tracheal tube over a fiber-optic bronchoscope (which has successfully been inserted into the trachea), are encountered while performing the mask ventilation, the risk of hypoxemia arises. Apneic oxygenation may be performed by the insufflation of oxygen through a RAE tube, a nasopharyngeal airway, or a short tracheal tube.
With HFNO, the nasal cannula does not need to be removed during attempts at laryngoscopy or fiber-optic bronchoscopy, or during the insertion of a supraglottic airway and this increases the stability of the oxygenation. Moreover, there is a low probability of the occurrence of problems that are associated with the incorrect positioning of the device, which are more likely to occur with a buccal RAE tube. This prevents the need for clinicians to abandon ongoing intubation attempts too early due to the occurrence of desaturation, and it allows for sufficient time for them to focus on performing the tracheal intubation and on any maneuvers required [43]. In addition, the nasal cannula used for HFNO does not interfere with the application of a facemask when additional mask ventilation is needed.
In 48 neurosurgical adult patients [10], the efficacy in increasing the PaO2 between HFNO with an oxygen flow of 50 L/min and an oxygen flow of 10 L/min through a facemask was compared. After 5 min of preoxygenation, the median PaO2 was significantly higher in the case of HFNO (471 mmHg) than in that of facemask oxygenation (357 mmHg). However, after the induction of anesthesia, the PaO2 decreased significantly in the HFNO group but not in the facemask group in which bag-mask ventilation was performed. This suggests that HFNO is more efficient than facemask oxygenation in ensuring a high PaO2 before the induction of anesthesia, but HFNO is less effective than facemask ventilation in maintaining a high PaO2. Nevertheless, 7 patients in the facemask group required the use of airway adjuncts such as oropharyngeal airways, but none of the patients in the HFNO group required them, suggesting that HFNO is more easily incorporated.
A male patient who had acute epiglottitis requiring emergency tracheal intubation [44] exhibited the following characteristics: an airway assessed as a Mallampati score of 2, a thyromental distance of > 6 cm, poor dentition, adequate mouth opening and neck range of movement, and an easily palpable cricothyroid membrane. Despite the administration of intravenous antibiotics and steroids, the airway obstruction progressed rapidly and required airway intervention. HFNO at a flow rate of 70 L/min was used in combination with the target-controlled infusion (TCI) of propofol, thereby allowing spontaneous breathing. The minimum oxygen saturation was 96% following tracheal intubation.
Oxygenation can be more difficult to perform in obese patients because the incidences of difficult mask ventilation and tracheal intubation may increase with obesity. The head-up position is helpful to maximize the efficacy of apneic oxygenation by reducing atelectasis and the subsequent pulmonary shunting [12,29]. Another study conducted on obese patients demonstrated that HFNO at a flow rate of 50 L/min resulted in the highest PaO2 value after 3–5 min of preoxygenation in morbidly obese patients and that the PaO2 decreased as the preoxygenation duration was extended up to 7 min [39]. Therefore, the appropriate duration of preoxygenation using HFNO remains to be determined.
Compared to adults, children are more prone to the risk of rapid desaturation in the order of seconds after the cessation of ventilation due to their decreased functional residual capacity, higher oxygen consumption, increased closing capacity, and higher risk of airway collapse [17,45]. A study has demonstrated that the mean desaturation time until the SpO2 reached 90% was significantly shorter in children (160 s) than in adolescents (382 s), and it was substantially shorter in infants (97 s). In fact, the incidence of desaturation is common in children (4–10% during induction of anesthesia and 20% during tracheal intubation) [18]. Therefore, in children, optimal oxygenation strategies are crucial in order to extend the duration of safe apnea.
HFNO may extend the safe apnea time in children. In a study involving 48 children who were under 10 years with normal airways [46], in the facemask group, bag-valve ventilation was ceased and the jaw thrust was maintained whereas in the HFNO group, the flow rates of 2 L/kg/min, 35, 40, and 50 L/min were applied based on the body weight, and the jaw thrust method was performed during apnea. The main hypothesis was that the safe apnea time (time taken for the SpO2 to reach 92%) in the case of HFNO would be more than double the length of the apnea time in the facemask group and the results confirm that this hypothesis was true.
In another study involving 60 children, aged 1–6 years, weighing 10–20 kg, HFNO at a flow rate of 2 L/kg/min with an FiO2 of 1.0, HFNO at a flow rate of 2 L/kg/min with an FiO2 of 0.3, and low-flow nasal oxygenation at a flow rate of 0.2 L/kg/min with an FiO2 of 1.0 were compared [47]. Bag-valve mask ventilation was ceased when the target EtO2 of > 90% was reached. Apnea was terminated when the SpO2 was less than 95%, transcutaneous carbon dioxide (tcCO2) reached 65 mmHg, or apnea duration reached 10 min. The SpO2 had decreased to < 95% within 10 min in all the HFNO (FiO2 0.3) patients and in 3 of the low-flow nasal oxygenation patients (17%), but it did not decrease in any of the HFNO (FiO2 1.0) patients, indicating that HFNO at a flow rate of 2 L/kg/min with an FiO2 of 0.3 is not effective. The tcCO2 exceeded above 65 mmHg within 10 min in 16 (80%) HFNO patients with an FiO2 of 1.0 and in 13 (72%) low-flow nasal oxygenation patients. In the case of HFNO with a flow rate of 2 L/kg/min and an FiO2 of 1.0, the median safe apnea time was 7.6 min with a range of 5.2–10 min. Therefore, in children, HFNO with a flow rate of 2 L/kg/min and an FiO2 of 1.0 can maintain the SpO2 for 10 min; however, there is a higher risk of hypercapnea with this technique, and therefore, it may be useful for surgeries that last between 5–6 min. It is not known whether or not a higher flow rate (e.g., 4 L/kg/min) with an FiO2 of less than 1.0 can be effective.
HFNO may also have a potential role during rapid-sequence induction of anesthesia in the operating room. In a study involving 80 adults who were receiving rapid-sequence induction of anesthesia, the efficacy of oxygenation was compared between HFNO at a flow rate of 70 L/min and facemask oxygenation at a rate of 10 L/min [36]. The lowest SpO2 up to 1 min after tracheal intubation was similar between the techniques, but the SpO2 decreased to < 96% in 7 patients (18%) in the facemask group whereas this was not observed in the HFNO group. None of the patients developed complications, such as the regurgitation of gastric contents, and there was no significant difference in the duration of the safe apnea time between HFNO (median: 116 s) and facemask (median: 109 s).
In another study involving 40 adult patients receiving rapid-sequence induction of anesthesia for emergency surgery, the efficacy between HFNO at the rate of 70 L/min and facemask oxygenation at the rate of 12 L/min were compared [48]. In the HFNO group, HFNO was maintained throughout the induction period whereas in the facemask group, the jaw was kept thrust out without bag-mask ventilation. In all the patients, the trachea was intubated successfully and there were no significant differences in the PaO2 between the groups. Nevertheless, it took significantly longer to intubate the trachea in the HFNO group (the mean of 248 s) than in the facemask group (123 s). The authors of the report state that this difference was not due to any apparent difference in procedural difficulty; however, the difference was quite marked. One of the reasons might be that the presence of a nasal cannula mechanically or psychologically facilitated a more careful time-consuming laryngoscopy and tracheal intubation.
These results indicate that HFNO with the jaw thrust is an efficient method to prevent desaturation within 3–4 min of apnea during rapid-sequence induction of anesthesia in adults even if cricoid pressure is being applied.
Securing the airway is frequently more difficult outside the operating rooms than inside [49,50]. This is because outside the operating room, the unstable physiological status of patients, such as being affected by a cardiopulmonary disease, having a low cardiac output, or being in hypermetabolic states, limits the efficiency of preoxygenation and peroxygenation. In addition, emergency procedures, poor planning, less skilled staff, and the inadequate availability of equipment increase the incidence of difficult intubation to up to approximately 12% [43,51,52]. Therefore, tracheal intubation is associated with a high incidence of hypoxemia, which is the most commonly associated complication (approximately 19–26%) [51,53] that is linked to hemodynamic deterioration, cardiac arrest, and death [12,54]. The incidence of death related to airway management has been reported to be 38-fold higher in the emergency department and 58-fold higher in the ICU than in the operating rooms [12].
Meta-analyses have shown that oxygenation through a nasal cannula at various flow rates is effective in preventing desaturation in patients who received emergency tracheal intubation [20,43]. In a study involving 101 adult patients who required rapid-sequence induction of anesthesia and intubation in the ICU [52], the efficacy of HFNO was compared with that of a non-rebreathing bag reservoir facemask combined with a nasopharyngeal catheter. For the HFNO group, HFNO at a flow rate of 60 L/min with an FiO2 of 1.0 was used for 3 min before the induction of anesthesia (preoxygenation) and was continued after the induction of anesthesia and during tracheal intubation. For the facemask group, 15 L/min of oxygen was provided for at least 3 min before the induction of anesthesia, and after the induction, the facemask was removed, a nasal cannula was placed, and 6 L/min of oxygen was insufflated. The median of the lowest SpO2 was significantly higher in the HFNO group (100%, range: 95–100%) than in facemask group (94%; 83–99%). Episodes of severe hypoxemia (SpO2 < 80%) were significantly lower in the HFNO group (2%) than in facemask group (14%); further, 1 cardiac arrest due to hypoxemia occurred in the facemask group. A multivariate analysis indicated that HFNO was an independent factor that prevented severe hypoxemia.
Nevertheless, HNFO may not be effective in improving oxygenation when tracheal intubation is required in hypoxemic patients. In a study involving 119 patients who had acute hypoxemic respiratory failure that required rapid-sequence induction and intubation (respiratory rate > 30 breaths/min, FiO2 requirement > 50%, PaO2/FiO2 < 300 mmHg) [55], either HFNO at a flow rate of 60 L/min with an FiO2 of 1.0 or facemask oxygenation at a flow rate of 15 L/min was used. There was no significant difference in the lowest SpO2 level (92% in HFNO group and 90% in facemask group) and in the occurrence of severe hypoxemia involving an SpO2 < 80% during tracheal intubation (16 [26%] in the HFNO group and 13 [22%] in the facemask group).
Similarly, another study that compared the efficacy of HFNO at a flow rate of 50 L/min and that of bag-valve-mask ventilation at a flow rate of 10 L/min in 40 critically ill patients who suffered hypoxemic respiratory failure (PaO2/FiO2 < 300 mmHg) [34], the lowest mean SpO2 during tracheal intubation and the occurrence of severe hypoxemia (SpO2 < 80%) were similar between the groups.
In a report on 49 adult patients who required rapid-sequence induction due to severe acute hypoxemic respiratory failure in the ICU (respiratory rate > 30 breaths/min, FiO2 requirement ≥ 50%, PaO2/FiO2 < 300 mmHg) [56], the efficacy of preoxygenation with HFNO at a flow rate of 60 L/min with an FiO2 of 1.0 together with non-invasive ventilation was compared with that of non-invasive ventilation performed on its own. HFNO plus non-invasive ventilation resulted in improved oxygenation and less frequent episodes of desaturation (SpO2 < 80%). Therefore, HFNO combined with non-invasive ventilation can reduce the incidence of severe hypoxemia during rapid-sequence induction of anesthesia in patients with severe acute hypoxemic respiratory failure.
In a prospective observational study that investigated the efficacy of HFNO at a flow rate of 60 L/min during emergency tracheal intubation in 71 adult patients in the ICU, in the operating room, or in the emergency department, the median (range) apnea time was 80 (30–480) s [37]. Significant desaturation (reduction in SpO2 to > 10% after induction of anesthesia) occurred in 5 patients (7%) who underwent acute respiratory failure (2 patients) or who had difficulty maintaining airway patency during apnea (3 patients). There were no complications resulting from the use of HFNO.
In the ICU, patients’ conditions in relation to their respiratory diseases and the techniques used in the control and intervention groups are varied. The intubation protocol including intubation equipment, patient positioning, preoxygenation approach, operators, and medications administered have not been standardized [57]. This may be a reason for the discrepancies among out-of-hospital studies. Moreover, the definitions of clinical outcomes vary between studies. For example, the standard that is taken into consideration to define hypoxemia is usually the SpO2 value, which is the starting point of the steep slope on the oxygen dissociation curve of hemoglobin [58]. Nevertheless, desaturation can be defined differently, which may be another reason for the conflicting results. Therefore, the efficacy of apneic HFNO compared to that of low-flow nasal apneic oxygenation is still unclear in emergency airway management in the ICU [3,20]. At least it is now apparent that HFNO alone may not be beneficial to patients who suffer severe respiratory failure [34,55]. This may be because pulmonary shunting or easily collapsed airways diminish the benefits of HFNO. To overcome this limitation, other techniques that provide high positive airway pressure may be required [58].
One major potential problem in relation to apneic oxygenation via HFNO is excessive hypercapnea. A study has demonstrated that the partial pressure of carbon dioxide (PaCO2) increased with the speed of 3 mmHg/min during apneic oxygenation via HFNO at a flow rate of 50 L/min [10]. The PaCO2 was significantly higher during apnea with HFNO than during bag-mask ventilation, but the resultant rise in the PaCO2 with HFNO seemed tolerable because the highest PaCO2 value was approximately 65 mmHg after the completion of tracheal intubation. Flow rates of up to 70 L/min may be required to achieve the maximum clearance of carbon dioxide. In contrast, carbon dioxide accumulation was similar between HFNO and facemask preoxygenation during rapid-sequence induction during emergency surgery [36,48] potentially due to a short observation period and the increase in carbon dioxide at a more rapid rate during the first minute following the onset of apnea. The clearance of carbon dioxide during HFNO may be superior to that resulting from a low-flow oxygen delivery system [59].
In children aged up to 10 years [46], the mean tcCO2 that was measured at the end of apnea was 62 mmHg (range 49–79 mmHg), and the mean rate of the increase in carbon dioxide was 2.4 mmHg/min (range 0.2–3.9 mmHg/min). However, the rate of increase in carbon dioxide was not significantly lower when HFNO was combined with the jaw thrust method compared to when jaw thrust was used in isolation during apnea. In a study involving children aged up to 6 years [47], the tcCO2 increased at a median rate (range) of 4.1 (2.2–5.3) kPa/min in response to HFNO with an FiO2 of 1.0, and the rate of increase in the tcCO2 was similar to that observed in the low-flow nasal oxygenation group. It has also been demonstrated that the younger the child, the faster the increase in the tcCO2 during apnea. These results imply that the efficiency in carbon dioxide clearance via HFNO is not prominent in children. This may be because the increase in carbon dioxide is higher in children than in adults and in younger children than in older children due to their higher metabolic demands.
The application of HFNO interrupts the earlier detection of a rise in carbon dioxide and of airway obstruction compared to when bag-mask ventilation is used. Therefore, the transcutaneous monitoring of carbon dioxide [10,46] and the use of oxygen reserve index sensors may help in minimizing this risk and in optimizing the utilization of HFNO.
Gastric insufflation is a theoretical complication resulting from HFNO because HFNO generates positive airway pressure. The increase in the nasal flow rate to 10 L/min is known to cause a 1.2 cmH2O increase in the nasopharyngeal airway pressure, as observed when healthy volunteers breathed with their mouths closed when HFNO was being used [4], and thus, the airway pressure would increase to around 3 cmH2O at 30 L/min and to around 12 cmH2O at 100 L/min.
No serious complications, such as gastric insufflation, regurgitation, or pulmonary aspiration of gastric contents, have so far been reported even in the case of morbidly obese patients [36,39]. The number of studies on this subject is still limited, and thus, the true risk of gastric insufflation associated with the use of HFNO during anesthesia remains to be elucidated.
Reports on the use of HFNO have indicated various high-flow rates such as 50 L/min [10,34,35,39], 60 L/min [32,37,52,55,56], or 70 L/min [33,36,48]. One study has demonstrated that the median intratracheal FiO2 significantly increased from 67% to 93% as the flow rate increased from 15 L/min to 45 L/min [60]. Similarly, another study has demonstrated that increasing the flow rate of HFNO from 10 L/min to 50 L/min can yield a higher FiO2 [61]. Therefore, using a higher flow rate of greater than 50 L/min is advisable to obtain the maximal effects of oxygenation.
Generally, HFNO is well tolerated by patients who are awake [35,36], but some of them may experience moderate or severe discomfort when a flow of 50–70 L/min is used [10,33]. Therefore, it may be prudent to start HFNO with a relatively low flow rate (30–40 L/min) when a patient is awake and then to increase the flow rate (50–70 L/min). When a patient cannot tolerate a high flow, the flow should immediately be reduced to 30–40 L/min, and once the patient has lost consciousness, the flow should be increased back to 50–70 L/min [10,35,36,48]. Concerning children, the adequate flow rate has been indicated to be 2 L/kg/min for those weighing 0–15 (or 20) kg, 35 L/min for those weighing 15 (or 20) –30 kg, 40 L/min for those weighing 30–50 kg, and 50 L/min for those weighing > 50 kg [46,47].
As the FiO2 increases, the safe apnea time gets extended. In addition, a study has demonstrated that an increase in the FiO2 from 0.9 to 1.0 yielded a greater increase in the safe apnea time compared to an increase in the FiO2 from 0.21 to 0.9 [62]. Therefore, the FiO2 should usually be set to 1.0 in order to maximize the safe apnea time in adults including in morbidly obese patients [39] and in those requiring rapid-sequence induction of anesthesia [36,52].
In children, HFNO with an FiO2 of 0.3 may not be as efficient in extending the safe apnea time when compared to HFNO with an FiO2 of 1.0 [47]; therefore, when HFNO is used, the FiO2 should be kept high.
There is still little evidence with regard to which breathing method is the best. In some studies, patients were asked to maintain normal breathing [32,33] whereas in other studies, deep breathing was preferred [35]. One clear characteristic is that the efficacy of HFNO in oxygenation is reduced when the patient’s mouth is open [2]. In a study [63] in which HFNO with a flow rate of 35 L/min was applied in postoperative cardiac surgery adult patients, the mean nasopharyngeal positive airway pressure was 2.7 cmH2O when the patient’s mouth was closed whereas it was only 1.2 cmH2O when the mouth was open. Similarly, in another study [64], the increase in the flow rates of HFNO resulted in increased mean airway pressures of 0.7 cmH2O per every 10 L/min when the mouth was closed, and the increased rate of airway pressure significantly reduced to 0.4 cmH2O when the mouth was open. Further [60], the median of mean airway pressure significantly increased from 0.4 cmH2O to 2 cmH2O as the oxygen flow was increased from 15 L/min to 45 L/min, but significantly decreased from 2 cmH2O to 0.6 cmH2O when the mouth was open. However, the FiO2 was not significantly different between when the mouth was closed or open with HFNO at a flow rate of 45 L/min [60]. In a study in which the EtO2 was measured during HFNO at a flow rate of 60 L/min [32], HFNO increased the EtO2 when the patient’s mouth was closed (86 kPa) whereas it failed to increase the EtO2 when the patient’s mouth was open (49 kPa).
Another crucial point to consider during HFNO is that the patency of the upper airway is essential to provide adequate oxygenation [17]. The patency of the upper airway needs to be maintained using a head tilt [37], jaw thrust [36,37,46–48], chin lift [36,37], or the insertion of a pharyngeal airway [47].
Awake tracheal intubation is another technique for securing the airway for which HFNO may be valuable. Patients undergoing awake fiber-optic intubation are at risk of desaturation due to underlying airway diseases, obesity, or sudden complete airway obstruction that can be caused by oversedation or topicalization [65]. The use of HFNO has a theoretical advantage with regard to providing oxygenation during awake fiber-optic intubation. This was confirmed by an observational study involving 50 adult patients [66] in which HFNO at a flow rate of 50–70 L/min was used during awake fiber-optic intubation. HFNO increased the median (range) of SpO2 from the baseline value of 98 (83–100)% to 100 (93–100)%, and none of the patients exhibited desaturation below the baseline SpO2 during the procedure. The median (range) of the EtCO2 was 4.8 (3.5–6.7) kPa after securing the airway. HFNO was also successfully used during surgical tracheostomy performed on a sedated patient with upper airway obstruction secondary to an infective acute leukemic mass [67].
Upper airway surgery often requires repetitive tracheal intubations and extubations during the procedure to allow access to the surgical field and to allow the manipulation required. It is therefore necessary to use specialized methods to reduce stressful conditions by extending the safe apnea time and by preventing hypoxemia [68]. There are several oxygenation techniques for airway surgery, such as transtracheal or transglottic jet ventilation, controlled mechanical ventilation using a small sized tracheal tube, and intermittent apneic ventilation [69]. Among these techniques, jet ventilation is the least preferred compared to the other techniques due to a resulting higher risk of barotrauma. The ideal method may be the tubeless technique that enables an optimal view of the surgical field and prevents tracheal tube-related injuries to the airways. The use of HFNO is a revolutionary oxygenation technique for airway surgery and it may replace the use of a tracheal tube during laryngomicrosurgery (Table 2).
Patel and Nouraei [59] were the first to report the use of HFNO during airway surgery. They used the technique in 25 adult patients who were undergoing hypopharyngeal or laryngotracheal surgeries, such as for benign laryngeal conditions, for obstructive sleep apnea, or for benign or malignant head and neck conditions. Their study included 12 obese patients and 9 patients with stridor. The median BMI (range) was 30 (18–52) kg/m2. The median apnea time was 14 min, with the longest duration being 65 min. None of the patients exhibited desaturation, i.e., an SpO2 of < 90%, when HFNO at a flow rate of 70 L/min was used. Further, none of the patients experienced complications, such as cardiac arrhythmia, due to an increase in carbon dioxide during apnea.
Booth et al. [70] employed a ‘traditional’ use of HFNO during airway surgery by maintaining spontaneous breathing. In 30 adult patients who underwent laryngotracheal surgeries due to laryngotracheal stenosis or papilloma, HFNO at a rate of 70 L/min was applied while anesthesia was maintained with TCI propofol, thereby maintaining spontaneous breathing. The median duration of spontaneous breathing (range) was 44 (18–100) min. Only 1 patient experienced desaturation due to a miscalculated remifentanil overdose, and tracheal intubation was required during the surgery. In another 3 patients, the SpO2 decreased to less than 90% during laser surgery when the FiO2 was reduced to 0.3. Nevertheless, the SpO2 increased rapidly when the FiO2 was increased back to 1.0.
In another report on 28 adult patients who were undergoing airway surgeries, apneic oxygenation was provided via a unique system called the Perioperative Insufflatory Nasal Therapy system at a flow rate of 80 L/min [71]. In 4 patients, the SpO2 decreased to 85–90%, lasting for less than 2 min at several time points, following the injection of rocuronium. The oxygen saturation was increased by the jaw thrust method and by increasing the flow rate to 120 L/min following the removal of the suspension laryngoscope or the insertion of a supraglottic airway. A different HFNO system (Optiflow) has a maximal flow rate of only 70 L/min; therefore, the rescue technique of increasing the flow rate to up to 120 L/min is not feasible with this system.
HFNO has also been successfully used in patients with difficult airways. For example, in a 55-year-old male who had severe supraglottic-pharyngeal stenosis, HFNO with a flow rate of 70 L/min and an FiO2 of 1.0 was applied, and the patient remained apneic for 26 min with the use of propofol, remifentanil, and rocuronium [72]. After the scar tissue was transected along the lateral epiglottic border resulting in the partial release of the stenotic lesion, the laser-safe tracheal tube was inserted through the opening. Until the time point at which the tracheal tube was inserted, the SpO2 was maintained at greater than 90%. In another report on a male patient who was morbidly obese with a BMI 40 kg/m2 and who had a neck circumference of 45 cm, mouth opening of 2 finger breaths, and Mallampati score of 4, HFNO at a flow rate of 60 L/min allowed for the performance of a 14-min long surgery without desaturation occurring [73].
HFNO may also be used in children, including in premature babies, who are undergoing airway surgeries. For example, a report describes the successful use of HFNO (at the flow rate of 2 L/kg/min) in more than 30 children (aged 6 days to 13 years) undergoing airway surgeries (e.g., aryepiglottoplasty, subglottic cyst excisions, tracheal dilatations, and endoscopic cricoid splints) [74]. The FiO2 was titrated to maintain the SpO2 at 95–99%, and spontaneous breathing was successfully preserved during surgery.
A study reported on a premature male baby who had a subglottic web after repeated tracheal intubations. He had severe inspiratory stridor that required laser resection and dilatation under general anesthesia [68]. Anesthesia was induced with sevoflurane and maintained with an infusion of propofol and remifentanil. Rocuronium was used to induce a full neuromuscular blockade, and an uncuffed tube was inserted. The surgeon placed the Parsons laryngoscope using the suspension apparatus. For the resection of the subglottic web, the trachea was extubated until the SpO2 fell to 80%, following which it was re-intubated. The apnea time was 39 and 41 s; however, it could be increased to 95 and 160 s when HFNO with a flow rate of 4 L/kg/min and an FiO2 of 0.3 was applied. When HFNO with an FiO2 of 1.0 was used, the apnea time extended to over 4 min. Following 2–4 min of apnea during HFNO, the EtCO2 was measured as 48–66 mmHg. This case report indicates that HFNO is efficient in extending the safe apnea time in an infant undergoing laryngeal repair even with a low FiO2 of 0.3.
Several complications associated with the use of HFNO during airway surgery have been reported.
One of the major complications associated with the use of HFNO during airway surgeries is the failure to maintain oxygenation. For example, in a report on the use of HFNO at a flow rate of 50 L/min during laryngomicrosurgery [75], the SpO2 decreased to 72% in 1 of the 23 studied patients, and tracheal intubation was temporarily required. In this case, HFNO was restarted, and the surgery could be completed without further complications.
In 20 infants and children (aged between 5 days to 15 years) who were undergoing upper airway surgeries or dynamic airway assessments lasting between 3–61 min, oxygenation was maintained via HFNO, but 1 of the neonates required rescue tracheal intubation at 3 min following apnea due to desaturation of 77% [76]. In a 35-year-old man with a parapharyngeal mass and a narrowing of the trachea to 4 mm, an emergency surgical tracheostomy was performed under sedation while HFNO at a flow rate of 30 L/min was applied with the patient in a 40° headup position [77]. When the sedation level was deepened, the respiratory rate decreased, and complete airway obstruction occurred. The SpO2 rapidly decreased to 89%, and an immediate tracheostomy was required. This case indicates that if the airway is obstructed, HFNO could delay but not prevent the onset of hypoxemia.
Hypercapnea is another major complication that occurs during airway surgeries because unlike short periods of apneic oxygenation before tracheal intubation, apneic oxygenation during airway surgery could last for as long as 40–60 min. There have been some reports on excessive hypercapnea requiring tracheal intubation or jet ventilation [78]. For example, in a case series on 30 non-obese patients who underwent elective short laryngeal procedures, HFNO at a flow rate of 70 L/min was applied while the patients were under apnea during surgery, and the surgeon inserted a rigid tubular laryngoscope [79]. In 1 of the patients, HFNO had to be stopped, and supraglottic jet ventilation was required because the PaCO2 exceeded a predetermined threshold of 11 kPa.
Compared with low-flow oxygenation, HFNO enables a greater clearance of carbon dioxide [71]. When HFNO (70–80 L/min) is used in adults for apneic oxygenation, the EtCO2 increases at a rate of 0.12–0.17 kPa/min [59,71,79]. By maintaining spontaneous breathing, the increase in EtCO2 can be reduced further (e.g., 0.03 kPa/min) [70].
The increase in EtCO2 progresses much faster in children than in adults. For example, in children weighing 10–20 kg, the mean rate of increase in tcCO2 was 0.55 kPa/min [47]. Younger children exhibited a more rapid carbon dioxide increase [47]. In a case report on infants weighing 4 kg, the EtCO2 increased from 35 mmHg to 51–52 mmHg after 250–251 s of apnea with HFNO at a rate of 4 L/kg/min [68].
One feature of the increase in carbon dioxide during apnea is that for an initial 1–2 min, the amount of carbon dioxide increases rapidly and then slows down [80]. For example, in a study [80], apnea increased the PaCO2 at a rate of 12 mmHg/min in the first minute, followed by a slow rise to 3.4 mmHg/min.
The exact mechanism of carbon dioxide clearance during HFNO is unclear although cardiogenic oscillations, dead space gas mixing, and micro-ventilation induced by pharyngeal pressure variations appear to be important factors [81].
Airway fire is a well-known complication that can occur during head and neck surgeries using lasers. Ignition sources, such as diathermy or laser rays, may come into accidental contact with inflammable items, such as tracheal tubes or surgical drapes, in an oxygen rich environment, and surgical fire can occur [82]. If HFNO is used during laser airway surgeries, a high concentration of oxygen flows through the surgical field of the airway, making it easier to ignite when the electrosurgical devices come into contact with this fuel. A case report on a 65-yearold female patient has been presented [83]. She was scheduled to receive a palatal biopsy. She had titanium dental implants in order to attach a palatal obturator following a previous surgery. HFNO at a rate of 30 L/min and an FiO2 of 1.0 was used while she was spontaneously breathing following the administration of propofol and fentanyl. A burn occurred on the diathermy shaft when the surgeon used a diathermy device to achieve hemostasis at the biopsy site. Fortunately, the tissue of the patient was not harmed. It is recommended that during the use of HFNO, the FiO2 should be lowered to the minimum level [71]. One possible method is to titrate the FiO2 to 0.3 using a gas blender [70]. It is recommended that clinicians be constantly vigilant concerning the risk of airway fires during laser or diathermy procedures.
When it is planned that HFNO is going to be employed for oxygenation during airway surgeries, special care needs to be taken with regard to the anesthesia method, airway patency, breathing mode, oxygen concentration, flow rate, and topicalization of the airway in order to maximize the margin of safety.
Anesthesia needs to be maintained with intravenous anesthesia techniques, such as the TCI of propofol [44,70,72,73,75,79] or its continuous infusion [59,68,71,76,78], because inhalational anesthetic agents cannot be administered via the HFNO system as they would be washed out with the high flow of gases, thereby not facilitating the adequate depth of anesthesia [84].
The reported flow rates of HFNO vary from 50 L/min [75] to 80 L/min [44,59,70–73,78,79] in adults, and a flow rate ≥ 50 L/min appears to be adequate. In children, the flow rate should be adjusted based on the body weight [68,76]. The FiO2 may be adjusted, but it would be safer to start with 1.0.
When performing apneic oxygenation, it is necessary to make sure that the airway is not obstructed during airway surgeries. The jaw thrust method may frequently be required to maintain airway patency [59,70,71,78,79]. In the case of microlaryngeal surgery, the surgeon usually inserts a suspension laryngoscope around the glottis, and this device may help to maintain airway patency during surgery [68,70–72]. It may be useful for surgeons to insert a tubular laryngoscope to ensure that the dorsal part of the laryngeal inlet is open in order to allow oxygen flow [79]. It should be kept in mind, however, that suspension laryngoscopy is accompanied with an opened mouth, and this may limit the ability of HFNO to generate positive airway pressure [71].
HFNO may be used both in patients who are apneic [59,68,71–73,75,78,79] and in those who are breathing spontaneously [44,70,74,76,77]. The preservation of spontaneous breathing may permit a better control on the increase in carbon dioxide, airway patency, and oxygenation [44]. Additionally, spontaneous breathing may aid surgeons in locating the glottis by facilitating the confirmation of bubbling or the movement of the vocal cords even in the presence of severe laryngeal edema obscuring the anatomy. Spontaneous breathing could reduce the risk of neuromuscular blockade-induced complete airway obstruction.
To maintain spontaneous breathing, adequate titration of the sedative or anesthetic agents to maintain deep sedation and adequate topicalization of the upper airway are required; this makes sure that the patient can tolerate a surgery in the airway [70,74,76]. One method is to perform laryngoscopy and to spray a local anesthetic in the airway before performing the airway surgery [70]. The risk of airway reactivity increases when the neuromuscular blockade is not sufficient, topicalization of the airway is not adequate, and repeated attempts at the insertion of surgical instruments are required. In a case report on an infant undergoing laryngeal repair, it was outlined that the surgical procedure may require multiple attempts at intubation and extubation alongside a full neuromuscular blockade [68]. In addition, surgical stimulation varies constantly during a surgery, and sedative agents have to be titrated continuously in response to patient and surgical conditions; this may result in the sedation being too deep, which is associated with accidental and sudden apnea [70].
Acute respiratory acidosis due to an increase in carbon dioxide during apnea does not act as a significant risk [79]. In addition, moderate carbon dioxide accumulation of up to 100 mmHg is not associated with cardiac arrhythmia or sympathetic stimulation [59,85] whereas severe carbon dioxide retention of more than 100 mmHg is associated with delayed recovery, ICU admission, and complications, such as postoperative congestive heart failure [85]. Patients who are at a risk of carbon dioxide increase, such as those suffering from pulmonary hypertension, obstructive airway diseases, raised intracranial pressure, and cardiac dysrhythmia, may be excluded from the use of HFNO. As there is a strong correlation between the tcCO2 and arterial PaCO2, monitoring the tcCO2 should be useful to detect hypercapnea [78,79].
Clinicians should be aware of the possibility that desaturation could occur during surgery and must have a backup plan to perform other oxygenation techniques. One of the plans is the use of jet ventilation. In a report on a patient who had tracheobronchomalacia with a BMI of 34 kg/m2 and on another patient who had spasmodic dysphonia with a BMI of 52 kg/m2, desaturation occurred during surgery, but jet ventilation was successfully established to increase the saturation [59]. In another report [73], infraglottic jet insufflation via cannula cricothyroidotomy was considered in order to reverse desaturation as this method does not interfere with the ongoing surgery.
In emergencies such as acute epiglottitis [44] or supraglottic-pharyngeal stenosis [72], clinicians should always keep in mind the possibility of a failure of HFNO and a priori prepare for emergency tracheostomy. The front of the neck should be evaluated thoroughly preoperatively, and the surgeon should be immediately available in the event of complete airway obstruction. In a case series [78], tracheal intubation with a smaller tube or supraglottic jet ventilation was planned before apneic oxygenation and was performed when the tcCO2 reached 70 mmHg.
It seems reasonable to conclude that HFNO is a superior technique to conventional oxygenation techniques in adults and children who require tracheal intubation in the operating room (Fig. 3). Regarding specific populations, such as those who are obese, pregnant, or in need of rapid-sequence induction of anesthesia, HFNO may be a feasible technique for delaying the onset of hypoxemia. In contrast, the efficacy of the use of HFNO in the case of critically ill patients remains unclear. Because the number of reported clinical trials is still insufficient and because of conflicting results, the most adequate protocol and indications for the use of HFNO should be explored and standardized via larger clinical trials.
When the first attempt at tracheal intubation fails, an immediate decision should be made as to whether and when bagmask ventilation should be commenced. If a facemask is used during preoxygenation and if a nasal cannula is used only during the apneic periods in the case of a laryngoscopy until the tracheal tube insertion is completed, the nasal cannula may impair efficient preoxygenation via the facemask by introducing oxygen leaks and the entrainment of room air [86]. When a nasal cannula is used together with a facemask, oxygen insufflation at a flow rate of 10 L/min or greater is recommended [86].
HFNO may be the most relevant in patients who are going to undergo rapid-sequence induction of anesthesia, which involves a relatively short apneic period (i.e., 1 min), compared to in those who are scheduled to undergo a conventional technique for the induction of anesthesia in which bag-mask ventilation can be avoided [36,39]. If it takes more than 3–4 min to secure the airway and if bag-mask ventilation is appropriate, then it may be less useful to employ HFNO because the oxygen saturation may decrease further in response to passive apneic oxygenation compared to with active bag-mask ventilation [10]. Nevertheless, the majority of cases involving difficult oxygenation after the induction of anesthesia cannot be predicted preoperatively [87], and thus, some researchers suggest that HFNO should be carried out in all the patients who receive anesthesia [22]. At the same time, clinicians should be aware that HFNO is not an adequate approach for recovering the SpO2 when significant desaturation occurs. Therefore, before the induction of anesthesia, preoperative airway assessments should be performed meticulously, and an airway strategy should be developed with a backup plan.
It should be considered that the use of HFNO should be added to the difficult airway guidelines and that its areas of application should be expanded to special populations such as to those with reduced cardiopulmonary reserve, to children, and to obese and pregnant patients [88].
Adequate communication, understanding, and teamwork are essential in order to establish intraoperative oxygenation using HFNO during airway surgeries. HFNO should not preclude the use of an alternate oxygenation plan, which should be made preoperatively, to resolve significant desaturation, hypercarbia, and acidosis. Therefore, sufficient planning and preparation should be carried out prior to preoxygenation. For short-duration surgeries, an increase in carbon dioxide and not the oxygenation technique may be the factor that determines the duration of apnea. TcCO2 monitoring is recommended for long procedures that last for more than 30 min [89].
HFNO is a promising new technique that keeps patients safer during anesthesia. Despite the mounting evidence supporting its use, more research and clinical trials are needed in order to establish the ideal use of this technique in various populations. We hope that this review will help readers to understand the relevant techniques involved in the use of HFNO and to facilitate the introduction of its use into the Airway Management Guidelines in the future.
References
1. Asai T. Strategies for difficult airway management--the current state is not ideal. J Anesth. 2013; 27:157–60.

2. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018; 15:145–55.

3. Renda T, Corrado A, Iskandar G, Pelaia G, Abdalla K, Navalesi P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth. 2018; 120:18–27.

4. Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015; 60:1397–403.

5. Cortegiani A, Accurso G, Mercadante S, Giarratano A, Gregoretti C. High flow nasal therapy in perioperative medicine: from operating room to general ward. BMC Anesthesiol. 2018; 18:166.

6. Pillow JJ, Hillman NH, Polglase GR, Moss TJ, Kallapur SG, Cheah FC, et al. Oxygen, temperature and humidity of inspired gases and their influences on airway and lung tissue in near-term lambs. Intensive Care Med. 2009; 35:2157–63.

7. Zochios V, Collier T, Blaudszun G, Butchart A, Earwaker M, Jones N, et al. The effect of high-flow nasal oxygen on hospital length of stay in cardiac surgical patients at high risk for respiratory complications: a randomised controlled trial. Anaesthesia. 2018; 73:1478–88.

8. Lu Z, Chang W, Meng S, Xue M, Xie J, Xu J, et al. The effect of high-flow nasal oxygen therapy on postoperative pulmonary complications and hospital length of stay in postoperative patients: a systematic review and meta-analysis. J Intensive Care Med. 2018; 885066618817718.

9. Sotello D, Rivas M, Mulkey Z, Nugent K. High-flow nasal cannula oxygen in adult patients: a narrative review. Am J Med Sci. 2015; 349:179–85.

10. Ng I, Krieser R, Mezzavia P, Lee K, Tseng C, Douglas N, et al. The use of Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) for pre-oxygenation in neurosurgical patients: a randomised controlled trial. Anaesth Intensive Care. 2018; 46:360–7.

11. Hengen M, Willemain R, Meyer A, Langer B, Joshi GP, Diemunsch P. Transnasal humidified rapid-insufflation ventilatory exchange for preoxygenation before cesarean delivery under general anesthesia: a case report. A A Case Rep. 2017; 9:216–8.
12. Cook TM, MacDougall-Davis SR. Complications and failure of airway management. Br J Anaesth. 2012; 109 Suppl 1:i68–85.

13. Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015; 115:827–48.
14. Gagnon C, Fortier LP, Donati F. When a leak is unavoidable, preoxygenation is equally ineffective with vital capacity or tidal volume breathing. Can J Anaesth. 2006; 53:86–91.

15. Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anaesth. 2009; 56:449–66.

16. Pourmand A, Robinson C, Dorwart K, O'Connell F. Pre-oxygenation: implications in emergency airway management. Am J Emerg Med. 2017; 35:1177–83.

17. Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesth Analg. 2017; 124:507–17.
18. Bouroche G, Bourgain JL. Preoxygenation and general anesthesia: a review. Minerva Anestesiol. 2015; 81:910–20.
19. Tan P, Dennis AT. High flow humidified nasal oxygen in pregnant women. Anaesth Intensive Care. 2018; 46:36–41.

20. Tan E, Loubani O, Kureshi N, Green RS. Does apneic oxygenation prevent desaturation during emergency airway management? A systematic review and meta-analysis. Can J Anaesth. 2018; 65:936–49.

21. Mitterlechner T, Herff H, Hammel CW, Braun P, Paal P, Wenzel V, et al. A dual-use laryngoscope to facilitate apneic oxygenation. J Emerg Med. 2015; 48:103–7.

22. Grude O, Solli HJ, Andersen C, Oveland NP. Effect of nasal or nasopharyngeal apneic oxygenation on desaturation during induction of anesthesia and endotracheal intubation in the operating room: a narrative review of randomized controlled trials. J Clin Anesth. 2018; 51:1–7.

23. Wimalasena Y, Burns B, Reid C, Ware S, Habig K. Apneic oxygenation was associated with decreased desaturation rates during rapid sequence intubation by an Australian helicopter emergency medicine service. Ann Emerg Med. 2015; 65:371–6.

24. Sakles JC, Mosier JM, Patanwala AE, Dicken JM. Apneic oxygenation is associated with a reduction in the incidence of hypoxemia during the RSI of patients with intracranial hemorrhage in the emergency department. Intern Emerg Med. 2016; 11:983–92.

25. Ramachandran SK, Cosnowski A, Shanks A, Turner CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth. 2010; 22:164–8.

26. Hayes-Bradley C, Lewis A, Burns B, Miller M. Efficacy of nasal cannula oxygen as a preoxygenation adjunct in emergency airway management. Ann Emerg Med. 2016; 68:174–80.
27. Achar SK, Pai AJ, Shenoy UK. Apneic Oxygenation during simulated prolonged difficult laryngoscopy: Comparison of nasal prongs versus nasopharyngeal catheter: a prospective randomized controlled study. Anesth Essays Res. 2014; 8:63–7.

28. Toner AJ, Douglas SG, Bailey MA, Avis HJ, Pillai AV, Phillips M, et al. Effect of apneic oxygenation on tracheal oxygen levels, tracheal pressure, and carbon dioxide accumulation: a randomized, controlled trial of buccal oxygen administration. Anesth Analg. 2019; 128:1154–9.
29. Heard A, Toner AJ, Evans JR, Aranda Palacios AM, Lauer S. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of buccal rae tube oxygen administration. Anesth Analg. 2017; 124:1162–7.
30. Windpassinger M, Plattner O, Gemeiner J, Röder G, Baumann A, Zimmerman NM, et al. Pharyngeal oxygen insufflation during airtraq laryngoscopy slows arterial desaturation in infants and small children. Anesth Analg. 2016; 122:1153–7.

31. Taha SK, Siddik-Sayyid SM, El-Khatib MF, Dagher CM, Hakki MA, Baraka AS. Nasopharyngeal oxygen insufflation following preoxygenation using the four deep breath technique. Anaesthesia. 2006; 61:427–30.

32. Pillai A, Daga V, Lewis J, Mahmoud M, Mushambi M, Bogod D. High-flow humidified nasal oxygenation vs. standard face mask oxygenation. Anaesthesia. 2016; 71:1280–3.

33. Ang KS, Green A, Ramaswamy KK, Frerk C. Preoxygenation using the Optiflow™ system. Br J Anaesth. 2017; 118:463–4.

34. Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S. High-flow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care. 2016; 61:1160–7.

35. Tan PCF, Millay OJ, Leeton L, Dennis AT. High-flow humidified nasal preoxygenation in pregnant women: a prospective observational study. Br J Anaesth. 2019; 122:86–91.

36. Lodenius Å, Piehl J, Östlund A, Ullman J, Jonsson Fagerlund M. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. 2018; 73:564–71.

37. Doyle AJ, Stolady D, Mariyaselvam M, Wijewardena G, Gent E, Blunt M, et al. Preoxygenation and apneic oxygenation using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange for emergency intubation. J Crit Care. 2016; 36:8–12.

38. Berthoud MC, Peacock JE, Reilly CS. Effectiveness of preoxygenation in morbidly obese patients. Br J Anaesth. 1991; 67:464–6.

39. Heinrich S, Horbach T, Stubner B, Prottengeier J, Irouschek A, Schmidt J. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J Obes Bariatrics. 2014; 1:7.

40. Mushambi MC, Kinsella SM, Popat M, Swales H, Ramaswamy KK, Winton AL, et al. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia. 2015; 70:1286–306.

41. Quinn AC, Milne D, Columb M, Gorton H, Knight M. Failed tracheal intubation in obstetric anaesthesia: 2 yr national case-control study in the UK. Br J Anaesth. 2013; 110:74–80.

42. Pillai A, Chikhani M, Hardman JG. Apnoeic oxygenation in pregnancy: a modelling investigation. Anaesthesia. 2016; 71:1077–80.

43. Binks MJ, Holyoak RS, Melhuish TM, Vlok R, Bond E, White LD. Apneic oxygenation during intubation in the emergency department and during retrieval: a systematic review and meta-analysis. Am J Emerg Med. 2017; 35:1542–6.

44. Lee PK, Booth AWG, Vidhani K. Spontaneous respiration using intravenous anesthesia and high-flow nasal oxygen (STRIVE Hi) management of acute adult epiglottitis: a case report. A A Pract. 2018; 10:73–5.

45. Hardman JG, Wills JS. The development of hypoxaemia during apnoea in children: a computational modelling investigation. Br J Anaesth. 2006; 97:564–70.

46. Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. 2017; 118:232–8.

47. Riva T, Pedersen TH, Seiler S, Kasper N, Theiler L, Greif R, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. 2018; 120:592–9.

48. Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017; 72:439–43.

49. Higgs A, McGrath BA, Goddard C, Rangasami J, Suntharalingam G, Gale R, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018; 120:323–52.

50. Asai T. Airway management inside and outside operating rooms-circumstances are quite different. Br J Anaesth. 2018; 120:207–9.

51. Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008; 34:1835–42.

52. Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015; 43:574–83.

53. Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006; 34:2355–61.

54. Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth. 2004; 16:508–16.

55. Vourc'h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015; 41:1538–48.
56. Jaber S, Monnin M, Girard M, Conseil M, Cisse M, Carr J, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016; 42:1877–87.

57. Semler MW, Janz DR, Lentz RJ, Matthews DT, Norman BC, Assad TR, et al. Randomized trial of apneic oxygenation during endotracheal intubation of the critically Ill. Am J Respir Crit Care Med. 2016; 193:273–80.
58. Pavlov I, Medrano S, Weingart S. Apneic oxygenation reduces the incidence of hypoxemia during emergency intubation: a systematic review and meta-analysis. Am J Emerg Med. 2017; 35:1184–9.

59. Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015; 70:323–9.

60. Chanques G, Riboulet F, Molinari N, Carr J, Jung B, Prades A, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013; 79:1344–55.
61. Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011; 39:1103–10.

62. McNamara MJ, Hardman JG. Hypoxaemia during open-airway apnoea: a computational modelling analysis. Anaesthesia. 2005; 60:741–6.

63. Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009; 103:886–90.

64. Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011; 56:1151–5.

65. McGuire G, el-Beheiry H. Complete upper airway obstruction during awake fibreoptic intubation in patients with unstable cervical spine fractures. Can J Anaesth. 1999; 46:176–8.

66. Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth. 2015; 115:629–32.

67. Ffrench-O'Carroll R, Fitzpatrick K, Jonker WR, Choo M, Tujjar O. Maintaining oxygenation with high-flow nasal cannula during emergent awake surgical tracheostomy. Br J Anaesth. 2017; 118:954–5.
68. Riva T, Seiler S, Stucki F, Greif R, Theiler L. High-flow nasal cannula therapy and apnea time in laryngeal surgery. Paediatr Anaesth. 2016; 26:1206–8.

69. Jaquet Y, Monnier P, Van Melle G, Ravussin P, Spahn DR, Chollet-Rivier M. Complications of different ventilation strategies in endoscopic laryngeal surgery: a 10-year review. Anesthesiology. 2006; 104:52–9.
70. Booth AW, Vidhani K, Lee PK, Thomsett CM. SponTaneous Respiration using IntraVEnous anaesthesia and Hi-flow nasal oxygen (STRIVE Hi) maintains oxygenation and airway patency during management of the obstructed airway: an observational study. Br J Anaesth. 2017; 118:444–51.

71. Lyons C, Callaghan M. Apnoeic oxygenation with high-flow nasal oxygen for laryngeal surgery: a case series. Anaesthesia. 2017; 72:1379–87.

72. Tam K, Jeffery C, Sung CK. Surgical management of supraglottic stenosis using intubationless optiflow. Ann Otol Rhinol Laryngol. 2017; 126:669–72.

73. Lee SJ, Quek KH. Facilitating airway surgery in a morbidly obese patient using transnasal humidified rapid insufflation ventilatory exchange (THRIVE). Case Rep Anesthesiol. 2018; 2018:5310342.

74. McCormack JG, Krosnar S, Baxter A. Reply to 'Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial'. Br J Anaesth. 2017; 119:172.

75. Yang SH, Wu CY, Tseng WH, Cherng WY, Hsiao TY, Cheng YJ, et al. Nonintubated laryngomicrosurgery with transnasal humidified rapidinsufflation ventilatory exchange: a case series. J Formos Med Assoc. 2019; 118:1138–43.

76. Humphreys S, Rosen D, Housden T, Taylor J, Schibler A. Nasal high-flow oxygen delivery in children with abnormal airways. Paediatr Anaesth. 2017; 27:616–20.

77. Desai N, Fowler A. Use of transnasal humidified rapid-insufflation ventilatory exchange for emergent surgical tracheostomy: a case report. A A Case Rep. 2017; 9:268–70.
78. Ebeling CG, Riccio CA. Apneic oxygenation with high-flow nasal cannula and transcutaneous carbon dioxide monitoring during airway surgery: a case series. A A Pract. 2019; 12:366–8.

79. Gustafsson IM, Lodenius Å, Tunelli J, Ullman J, Jonsson Fagerlund M. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) - a physiological study. Br J Anaesth. 2017; 118:610–7.

80. Stock MC, Schisler JQ, McSweeney TD. The PaCO2 rate of rise in anesthetized patients with airway obstruction. J Clin Anesth. 1989; 1:328–32.
81. Laviola M, Das A, Chikhani M, Bates DG, Hardman JG. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br J Anaesth. 2019; 122:395–401.

83. Onwochei D, El-Boghdadly K, Oakley R, Ahmad I. Intra-oral ignition of monopolar diathermy during transnasal humidified rapidinsufflation ventilatory exchange (THRIVE). Anaesthesia. 2017; 72:781–3.

85. Cheng Q, Zhang J, Wang H, Zhang R, Yue Y, Li L. Effect of acute hypercapnia on outcomes and predictive risk factors for complications among patients receiving bronchoscopic interventions under general anesthesia. PLoS One. 2015; 10:e0130771.

86. McQuade D, Miller MR, Hayes-Bradley C. Addition of nasal cannula can either impair or enhance preoxygenation with a bag valve mask: a randomized crossover design study comparing oxygen flow rates. Anesth Analg. 2018; 126:1214–8.
87. Nørskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrøm LH. Diagnostic accuracy of anaesthesiologists' prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia. 2015; 70:272–81.
Fig. 1.
Recent areas of use for high-flow nasal oxygenation, including during the anesthetic induction period and intraoperative period.

Fig. 2.
Equipment for high-flow nasal oxygenation (OptiflowTM, Fisher & Paykel Healthcare, New Zealand). (A) OptiflowTM consists of a flow meter, humidifier, heating system, heated non-condensing circuit, nasal cannula, head strap, and an oxygen connector for gas supply. (B) Nasal cannula. (C) Humidifier and heating system (© Fisher & Paykel Healthcare 2018. Used with permission).
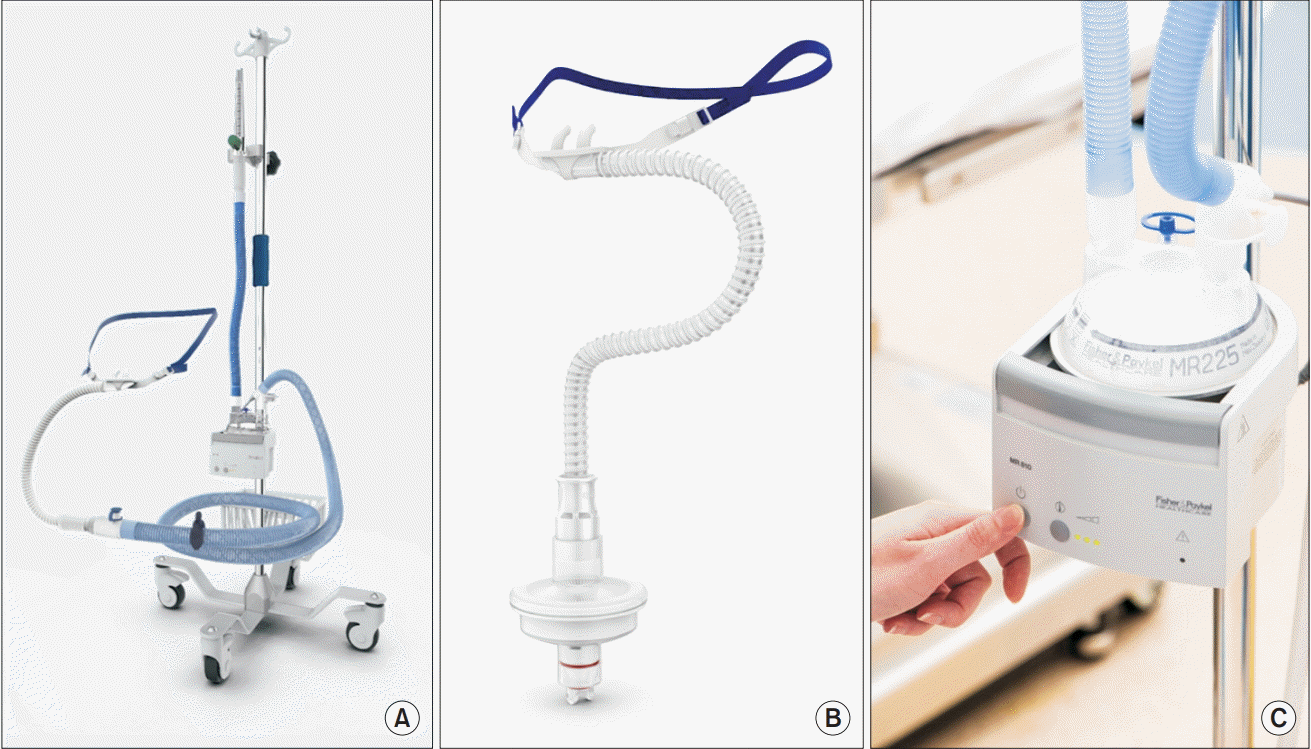
Fig. 3.
High-flow nasal oxygenation for tracheal intubation in the operating room. (A) Preoxygenation. (B) Apneic oxygenation (© Fisher & Paykel Healthcare 2018. Used with permission).
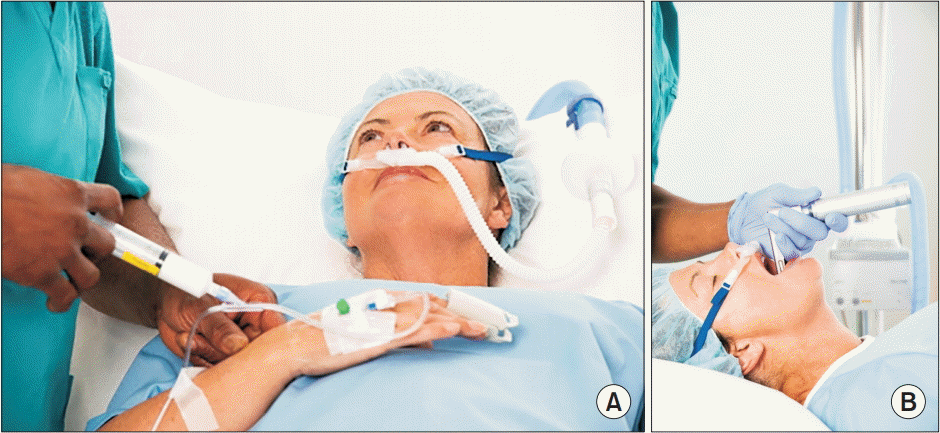
Table 1.
Characteristics of the Clinical Studies on High-flow Nasal Oxygenation (HFNO) Performed during the Induction of Anesthesia
| Year, author, design | Number of patients, location, inclusion (I), exclusion (E) | Intervention | Comparator | Outcome and results, Intervention group (I), Comparator (C) | |
|---|---|---|---|---|---|
| Preoxygenation | |||||
| Pillai et al. (2016), prospective study [32] | (n = 10) | (n = 10) | (n = 10) | 1) EtO2, mean (SD) (P = 0.001): | |
| I: healthy adult volunteers | OIA: HFNO at 60 L/min for 3 min (with mouth closed and open) | OIA: FM at 10 L/min for 3 min | I (mouth closed): 85.6 (6.4) kPa | ||
| E: respiratory or cardiac disease | I (mouth open): 48.7 (26.4) kPa | ||||
| C: 88.5 (6.2) kPa | |||||
| 2) Transcutaneous oxygen partial pressures, mean (SD) (P = 0.03): | |||||
| I (mouth closed): 36.4 (6.5) kPa | |||||
| I (mouth open): 25.5 (15.7) kPa | |||||
| C: 34.6 (5.4) kPa | |||||
| Ang et al. (2017), prospective pilot study [33] | (n = 21) | (n = 21) | No control | 1) EtO2, median (IQR) [range]: | |
| I: healthy adult volunteers | OIA: HFNO at 70 L/min for 30, 60, 90, 120, 150, and 180 s | I (30 s): 72% (66–79%) [45–82%] | |||
| I (60 s): 79% (71–86%) [65–89%] | |||||
| I (90 s): 84% (77–88%) [64–91%] | |||||
| I (120 s): 87% (80–91%) [72– 93%] | |||||
| I (150 s): 88% (83–90%) [75–94%] | |||||
| I (180 s): 86% (84–90%) [78–92%] | |||||
| Heinrich et al. (2014), prospective RCT [39] | (n = 33) | (n = 11) | (n = 11) | 1) PaO2, median (IQR): | |
| OR | OIA: HFNO at 50 L/min with FiO2 of 1.0 for 7 min (mouth closed) | OIA: FM at 12 L/min with FiO2 of 1.0 for 7 min | I: 380 (339–443) mmHg | ||
| I: morbidly obese adult patients scheduled for laparoscopic bariatric surgery. (BMI ≥ 35 kg/m2) | C: 337 (295–390) mmHg | ||||
| E: patients with severe pulmonary disorder, known or anticipated difficult airway | |||||
| Hengen et al. (2017), case report [11] | (n = 1) | (n = 1) | No control | 1) SpO2: | |
| OR | OIA: HFNO of 70 L/min with FiO2 of 1.0 for 5 min. | 98% during endotracheal intubation. | |||
| I: 27-year-old non-obese pregnant patient with acute respiratory distress syndrome and heart failure. | OIS, OIL: HFNO of 70 L/min with FiO2 of 1.0 | ||||
| Tan et al. (2019), prospective observational study [35] | (n = 73) | (n = 73) | No control | 1) EtO2, median (IQR) [range]: | |
| I: ASA 2 pregnant women | OIA: HFNO at 30 L/min for 30 s, then at 50 L/min for 150 s. (deep breathing, with mouth closed) | I: 91% (83–93%) [58–96%] | |||
| E: Patients with significant nasal pathology, severe cardiac or respiratory disease, preeclampsia, sepsis | |||||
| Apneic oxygenation | |||||
| Ng et al. (2018), prospective RCT [10] | (n = 48) | (n = 24) | (n = 24) | 1) PaO2, median (IQR) [range] (P = 0.01): | |
| OR | OIA: HFNO at 30 L/min for 30 s, then at 50 L/min for 270 s | OIA: FM 10 L/min for 5 min. | I: 471 (429–516) [185–550] mmHg | ||
| I: ASA 1–3 adult patients | C: 357 (324–450) [183–550] mmHg | ||||
| E: Patients with BMI > 35 kg/m2, known or anticipated difficult airway, rapid sequence induction, significant raised intracranial pressure, active nasal bleeding, base of skull fracture | |||||
| Lee et al. (2018), case report [44] | (n = 1) | (n = 1) | No control | SpO2 ≥ 96% | |
| Adult | OIA: HFNO at 50 L/min with FiO2 of 1.0 | EtCO2: 39 mmHg | |||
| Patients with emergent endotracheal intubation for acute epiglottitis | OIS, OIL: HFNO at 70 L/min with FiO2 of 1.0 (Spontaneous respiration, no neuromuscular relaxation) | ||||
| Humphreys et al. (2017), prospective RCT [46] | (n = 48) | (n = 24) | (n = 24) | 1) Apnea time mean (P < 0.001): | |
| OR | OIS: bag-mask ventilation for 3 min. | OIS: bag-mask ventilation for 3 min | I: 192 s (0–6 months), 237 s (7–24 months), 320 s (2–5 yr), 430 s (6–10 yr). | ||
| I: healthy children (age: 0–6 months, 7–24 months, 2–5 yr and 6–10 yr old) | OIL: HFNO 0–15 kg, 2 L/kg/min; 15–30 kg, 35 L/min; 30–50 kg, 40 L/min; and > 50 kg, 50 L/min | OIL: no oxygenation | C: 109.2 s (0–6 months), 147.3 s (7–24 months), 190.5 s (2–5 yr), 260.8 s (6–10 yr). | ||
| 2) SpO2, mean [range]: | |||||
| I: 99.6% [97–100%] | |||||
| C: 92% [92%] | |||||
| Riva et al. (2018), prospective RCT [47] | (n = 60) | (n = 20, FiO2 1.0) | (n = 20) | 1) Apnea time, median (IQR) [range] (P < 0.001): | |
| OR | OIS: bag-mask ventilation | OIS: bag-mask ventilation | I (FiO2 1.0): 7.6 (6.2–9.1) [5.2–10.0] min | ||
| I: ASA 1–2 children (age: 1–6 yr, weight: 10–20 kg) | OIL: HFNO at 2 L/kg/min with FiO2 of 1.0 (n = 20, FiO2 0.3) | OIL: HFNO 0.2 at L/kg/min with FiO2 of 1.0 | I (FiO2 0.3): 3.0 (2.4–3.7) [0.2–5.3] min | ||
| E: Patients with known or anticipated difficult airway, congenital heart or lung disease, obesity (BMI > 30 kg/m2), risk of aspiration | OIS: bag-mask ventilation | C: 6.9 (5.7–7.8) [2.8–10.0] min | |||
| OIL: HFNO at 2 L/kg/min with FiO2 of 0.3 | |||||
| Rapid sequence induction | |||||
| Lodenius et al. (2018), prospective RCT [36] | (n = 80) | (n = 40) | (n = 40) | 1) lowest SpO2, median (IQR) [range] (P = 0.097): | |
| OR | OIA: HFNO at 40 L/min, then at 70 L/min with FiO2 of 1.0 for 3 min. | OIA: FM at 10 L/min with FiO2 of 1.0 for 3 min. | I: 99% (99–100%) [96–100%] | ||
| I: Adult patients who required rapid sequence induction | OIS, OIL: HFNO at 70 L/min with FiO2 of 1.0 | OIS: FM at 10 L/min, no bagmask ventilation. | C: 99% (97–100%) [70–100%] | ||
| E: Patients with BMI > 35 kg/m2 | OIL: no oxygenation. | ||||
| Mir et al. (2017), prospective RCT [48] | (n = 40) | (n = 20) | (n = 20) | 1) PaO2, mean (SD) (P = 0.722): | |
| OR | OIA: HFNO at 30 L/min, then at 70 L/min for 3 min. | OIA: FM at 12 L/min for 3 min. | I: 43.7 (15.2) kPa | ||
| I: Adult patients who required rapid sequence induction | OIS, OIL: HFNO at 70 L/min | OIS: FM at 12 L/min, no bagmask ventilation. | C: 41.9 (16.2) kPa | ||
| E: Patients with severe respiratory disease | OIL: no oxygenation. | ||||
| Miguel-Montanes et al. (2015), prospective beforeafter study [52] | (n = 101) | (n = 51) | (n = 50) | 1) lowest SpO2, median (IQR) (P < 0.0001): | |
| ICU | OIA: HFNO at 60 L/min with FiO2 of 1.0 for 3 min | OIA: NRM at 15 L/min for 3 min. | I: 100% (95–100%) | ||
| I: Adult patients who required rapid sequence induction | OIS, OIL: HFNO at 60 L/min with FiO2 of 1.0 | OIS: NRM at 15 L/min | C: 94% (83–98.5%) | ||
| E: Patients with cardiac arrest, severe hypoxemia (defined as SpO2 < 95% under a NRM with an oxygen flow of 15 L/min), patients already receiving HFNO, and patients under NIV | OIL: nasopharyngeal catheter at 6 L/min | ||||
| Vourc’h et al. (2015), prospective RCT [55] | (n = 119) | (n = 62) | (n = 57) | 1) lowest SpO2, median (IQR) (P = 0.44): | |
| ICU | OIA: HFNO at 60 L/min with FiO2 of 1.0 for 4 min | OIA: FM at 15 L/min for 4 min | I: 91.5 % (80–96%) | ||
| I: Adult patients who required rapid sequence induction with acute hypoxemic respiratory failure | OIS, OIL: HFNO of 60 L/min with FiO2 of 1.0 | OIS: FM at 15 L/min | C: 89.5 % (81–95%) | ||
| E: Patients with cardiac arrest, asphyxia, nasopharyngeal obstacle, grade 4 glottis exposure on the Cormack–Lehane scale | OIL: no oxygenation | ||||
| Simon et al. (2016), prospective RCT [34] | (n = 40) | (n = 20) | (n = 20) | 1) lowest SpO2, mean (SD) (P = 0.56): | |
| ICU | OIA: HFNO of 50 L/min with FiO2 of 1.0 | OIA: bag-valve mask at 10 L/min | I: 89 (18)% | ||
| I: Adult patients who required rapid sequence induction with hypoxemic respiratory failure | OIS, OIL: HFNO of 50 L/min with of FiO2 1.0 | OIS: bag-valve mask at 10 L/min | C: 86 (11)% | ||
| E: Patients with nasopharyngeal obstruction or blockage, suspected or known difficult airway | OIL: no oxygenation | ||||
| Jabor et al. (2016), prospective RCT [56] | (n = 49) | (n = 25) | (n = 24) | 1) lowest SpO2, median (IQR) (P = 0.029): | |
| ICU | OIA: HFNO of 60 L/min with FiO2 of 1.0 with NIV for 4 min | OIA: NIV with FiO2 of 1.0 for 4 min | I: 100% (95–100) % | ||
| I: Adult patients who required rapid sequence induction with acute hypoxemic respiratory failure | OIS: HFNO at 60 L/min with FiO2 of 1.0 with NIV | OIS: NIV with FiO2 of 1.0 | C: 96% (92–99) % | ||
| E: Patients with cardiac arrest, nasopharyngeal obstruction, usual contraindications to NIV | OIL: HFNO at 60 L/min with FiO2 of 1.0 | OIL: no oxygenation. | |||
| Doyle et al. (2016), prospective observational study [37] | (n = 71) | (n = 71) | No control | 1) Incidence of desaturation (reduction of SpO2 > 10%): | |
| ICU, OR, ED | OIA: HFNO at 60 L/min for 3 min. | I: 5 patients (7%) | |||
| I: Adult patients requiring intubation | OIS, OIL: HFNO at 60 L/min | ||||
ASA: American Society of Anesthesiologists, BMI: body mass index, FiO2: fraction of inspired oxygen, FM: face mask oxygenation, HFNO: high-flow nasal oxygenation, ICU: intensive care unit, IQR: interquartile range, NIV: non-invasive ventilation, NRM: non-rebreathing bag reservoir face mask, OIA: oxygenation-in-awake, OIL: oxygenation-in-laryngoscopy, OIS: oxygenation-insedative state, OR: operating room, PaO2: partial pressure of arterial oxygen, RCT: randomized controlled trial, SpO2: peripheral capillary oxygen saturation, SD: standard deviation.
Table 2.
Characteristics of Clinical Studies on High-flow Nasal Oxygenation (HFNO) for Airway Surgery
| Year, author, design | Number of patients | Inclusion | Apnea or spontaneous relaxation, neuromuscular relaxation | Oxygenation | Time of apnea or spontaneous respiration | SpO2 and EtCO2 or PaCO2 |
|---|---|---|---|---|---|---|
| Patel and Nouraei (2015), case series [59] | 25 | Adult | Apnea. | Preoxygenation: HFNO at 70 L/min with FiO2 of 1.0 for 10 min | Median (IQR) [range]: 14 (9–19) [5–65] min | SpO2 ≥ 90% EtCO2, mean (SD) [range]: 7.8 (2.4) [4.9–15.3] kPa. |
| Surgeries for laryngotracheal stenosis, vocal fold pathology and obstructive sleep apnea, and benign and malignant hypopharyngeal obstruction | Rocuronium 0.5 mg/kg | Peroxygenation: HFNO at 70 L/min with FiO2 of 1.0 | ||||
| Booth et al. (2017), case series [70] | 30 | Adult | Spontaneous respiration | Preoxygenation: HFNO at 30 L/min with FiO2 of 1.0 for 1 min, then at 50 L/min for 2 min. | Median (IQR) [range]: 44 (40–49.5) [18–100] min | |
| Elective microlaryngoscopic surgery | No neuromuscular relaxation | Peroxygenation: HFNO at 70 L/min with FiO2 of 1.0. | ||||
| Lyon and Callaghan (2017), case series [71] | 28 | Adult | Apnea | Preoxygenation: HFNO at 80 L/min with FiO2 of 1.0 for 3 min. | Median (IQR) [range]: 19 (15–24) [9–37] min | SpO2 ≥ 85% EtCO2, median (IQR) [range]: 8.2 (7.2–9.4) [5.8–11.8] kPa. |
| Laryngeal or tracheal surgeries | Rocuronium | Peroxygenation: HFNO at 80 L/min with FiO2 of 1.0. | ||||
| Tam et al. (2017), case report [72] | 1 | Adult | Apnea | Preoxygenation: HFNO at 35 L/min with FiO2 of 1.0 for 3 min. | 26 min | SpO2 ≥ 90% |
| CO2 laser release of supraglottic pharyngeal stenosis | Rocuronium and succinylcholine | Peroxygenation: HFNO at 70 L/min with FiO2 of 1.0. | ||||
| Lee and Quek (2018), case report [73] | 1 | Adult | Apnea | Preoxygenation: HFNO at 20 L/min, then at 60 L/min with FiO2 of 1.0 for 15 min. | 14 min | SpO2 ≥ 98% |
| Morbid obesity | Rocuronium 0.3 mg/kg | Peroxygenation: HFNO at 60 L/min with FiO2 of 1.0 | PaCO2: 60 mmHg | |||
| Elective panendoscopy and biopsy of vocal cord lesion | ||||||
| McCormack et al. (2017), case series [74] | > 30 | Children | Spontaneous respiration | Preoxygenation and peroxygenation: HFNO at 2 L/kg/min. | 30–40 min | tcCO2 6.5–7 kPa |
| No neuromuscular relaxation | ||||||
| Riva et al. (2016), case report [68] | 1 | Premature baby boy weighed 4 kg with a corrected age between 43 and 46 weeks | Apnea | Peroxygenation: HFNO at 4 L/kg/min with FiO2 of 0.3 or 1.0. | Range: | SpO2 ≥ 80% |
| Laser resection and dilatation of subglottic web | Rocuronium | (FiO2 0.3) 95–160 s | EtCO2: 48–66 mmHg | |||
| (FiO2 1.0) 70–234 s | ||||||
| Yang et al. (2018), case series [75] | 23 | Adult | Apnea. | Preoxygenation: HFNO at 20 L/min with FiO2 of 1.0 for 5 min. | Mean (SD): 24.1 (6.4) min | SpO2, minimum and median: 72% and 100% |
| Elective laryngomicrosurgeries for vocal cord polyp, cyst, and laryngeal tumor biopsy. | Succinylcholine 1.5 mg/kg | Peroxygenation: HFNO at 50 L/min with FiO2 of 1.0. | ||||
| Humphreys et al. (2017), case series [76] | 20 | Children, age 5 days to 15 years | Spontaneous respiration | Preoxygenation and peroxygenation: HFNO | Median [range]: 32 [3–61] min | SpO2, minimum and mean: 77% and 96% |
| Upper airway surgery or dynamic airway assessment | No neuromuscular relaxation | (0–12 kg) at 2 L/kg/min | ||||
| (13–15 kg) at 30 L/min | ||||||
| (15–30 kg) at 35 L/min | ||||||
| (30–50 kg) at 40 L/min | ||||||
| (> 50 kg) at 50 L/min | ||||||
| Desai and Fowler (2017), case report [77] | 1 | Adult | Spontaneous respiration | Preoxygenation: HFNO at 30 L/min with FiO2 of 1.0 for 15 min. | 40 min | SpO2 ≥ 89% |
| Emergent surgical tracheostomy due to parapharyngeal abscess | No neuromuscular relaxation | Peroxygenation: HFNO at 30 L/min with FiO2 of 1.0. | ||||
| Ebeling and Riccio (2018), case series [78] | 3 | Adult | Apnea | Preoxygenation: HFNO at 60 L/min with FiO2 of 1.0 for 5 min. | (1) 15 min | SpO2 ≥ 97% |
| (1) Microdebridement of bilateral true vocal cord polyps and Reinke’s edema | Rocuronium | (2) 15 min | (1) tcCO2 70 mmHg | |||
| (2) Tracheal balloon dilation of subglottis stenosis | Peroxygenation: HFNO at 60 L/min with FiO2 of 1.0. | (3) 40 min | (2) tcCO2 70 mmHg | |||
| (3) Biopsy of vocal cord lesion | (3) tcCO2 89.4 mmHg | |||||
| Gustafsson et al. (2017), case series [79] | 31 | Adult | Apnea | Preoxygenation: HFNO at 40 L/min with FiO2 of 1.0 for 3 min. | Mean (SD): 22.5 (4.5) min | SpO2 > 91% |
| Elective short laryngeal procedures, such as microlaryngoscopy | Rocuronium 0.6 mg/kg | Peroxygenation: HFNO at 70 L/min with FiO2 of 1.0. |




 Citation
Citation Print
Print



 XML Download
XML Download