1. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808.
2. Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine (Phila Pa 1976). 1982; 7:319–30.

3. Vincent MB, Luna RA, Scandiuzzi D, Novis SA. Greater occipital nerve blockade in cervicogenic headache. Arq Neuropsiquiatr. 1998; 56:720–5.

4. Bovim G, Sand T. Cervicogenic headache, migraine without aura and tension-type headache. Diagnostic blockade of greater occipital and supra-orbital nerves. Pain. 1992; 51:43–8.

5. Young W, Cook B, Malik S, Shaw J, Oshinsky M. The first 5 minutes after greater occipital nerve block. Headache. 2008; 48:1126–8.

6. Binder WJ, Brin MF, Blitzer A, Schoenrock LD, Pogoda JM. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: an open-label study. Otolaryngol Head Neck Surg. 2000; 123:669–76.

7. Greher M, Moriggl B, Curatolo M, Kirchmair L, Eichenberger U. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. Br J Anaesth. 2010; 104:637–42.

8. Pingree MJ, Sole JS, Oʼ Brien TG, Eldrige JS, Moeschler SM. Clinical efficacy of an ultrasound-guided greater occipital nerve block at the level of C2. Reg Anesth Pain Med. 2017; 42:99–104.

9. Shim JH, Ko SY, Bang MR, Jeon WJ, Cho SY, Yeom JH, et al. Ultrasound-guided greater occipital nerve block for patients with occipital headache and short term follow up. Korean J Anesthesiol. 2011; 61:50–4.

10. Stovner Lj, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007; 27:193–210.

11. Natsis K, Baraliakos X, Appell HJ, Tsikaras P, Gigis I, Koebke J. The course of the greater occipital nerve in the suboccipital region: a proposal for setting landmarks for local anesthesia in patients with occipital neuralgia. Clin Anat. 2006; 19:332–6.

12. Lim HB, Hunt K. Anesthetic management for surgical placement of greater occipital nerve stimulators in the treatment of primary headache disorders. J Neurosurg Anesthesiol. 2007; 19:120–4.

13. Ambrosini A, Vandenheede M, Rossi P, Aloj F, Sauli E, Pierelli F, et al. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain. 2005; 118:92–6.

14. Bartsch T, Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002; 125:1496–509.

15. Lauretti GR, Corrêa SW, Mattos AL. Efficacy of the greater occipital nerve block for cervicogenic headache: comparing classical and subcompartmental techniques. Pain Pract. 2015; 15:654–61.

16. Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009; 123:859–63.

17. Cesmebasi A, Muhleman MA, Hulsberg P, Gielecki J, Matusz P, Tubbs RS, et al. Occipital neuralgia: anatomic considerations. Clin Anat. 2015; 28:101–8.

18. Graboski CL, Gray DS, Burnham RS. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomised double blind crossover study. Pain. 2005; 118:170–5.

19. Ho KY, Tan KH. Botulinum toxin A for myofascial trigger point injection: a qualitative systematic review. Eur J Pain. 2007; 11:519–27.

20. Kapural L, Stillman M, Kapural M, McIntyre P, Guirgius M, Mekhail N. Botulinum toxin occipital nerve block for the treatment of severe occipital neuralgia: a case series. Pain Pract. 2007; 7:337–40.

21. Ashkenazi A, Matro R, Shaw JW, Abbas MA, Silberstein SD. Greater occipital nerve block using local anaesthetics alone or with triamcinolone for transformed migraine: a randomised comparative study. J Neurol Neurosurg Psychiatry. 2008; 79:415–7.

22. Montecucco C. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 2005; 5:274–9.

23. Dressler D, Saberi FA, Barbosa ER. Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr. 2005; 63:180–5.

24. Flanders M, Tischler A, Wise J, Williams F, Beneish R, Auger N. Injection of type A botulinum toxin into extraocular muscles for correction of strabismus. Can J Ophthalmol. 1987; 22:212–7.
25. Bushara KO, Park DM, Jones JC, Schutta HS. Botulinum toxin--a possible new treatment for axillary hyperhidrosis. Clin Exp Dermatol. 1996; 21:276–8.

26. Clark RP, Berris CE. Botulinum toxin: a treatment for facial asymmetry caused by facial nerve paralysis. Plast Reconstr Surg. 1989; 84:353–5.

27. Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: mechanisms of action. Br J Pharmacol. 2014; 171:4177–92.

28. Lew MF. Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clin J Pain. 2002; 18(6 Suppl):S142–6.

29. Afridi SK, Shields KG, Bhola R, Goadsby PJ. Greater occipital nerve injection in primary headache syndromes--prolonged effects from a single injection. Pain. 2006; 122:126–9.
30. Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005; 53:407–15.
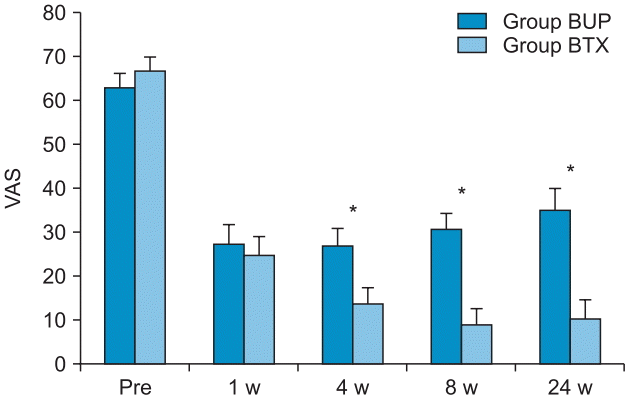
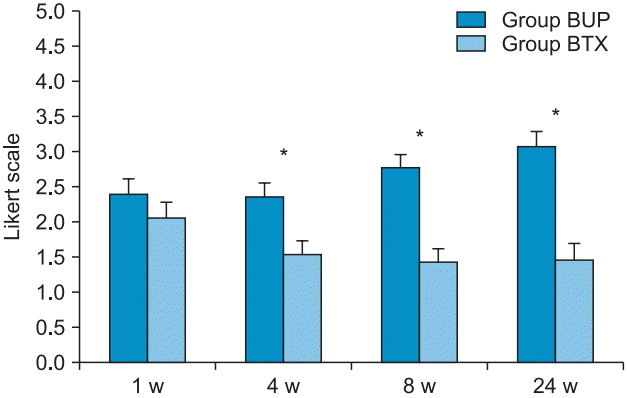




 PDF
PDF Citation
Citation Print
Print



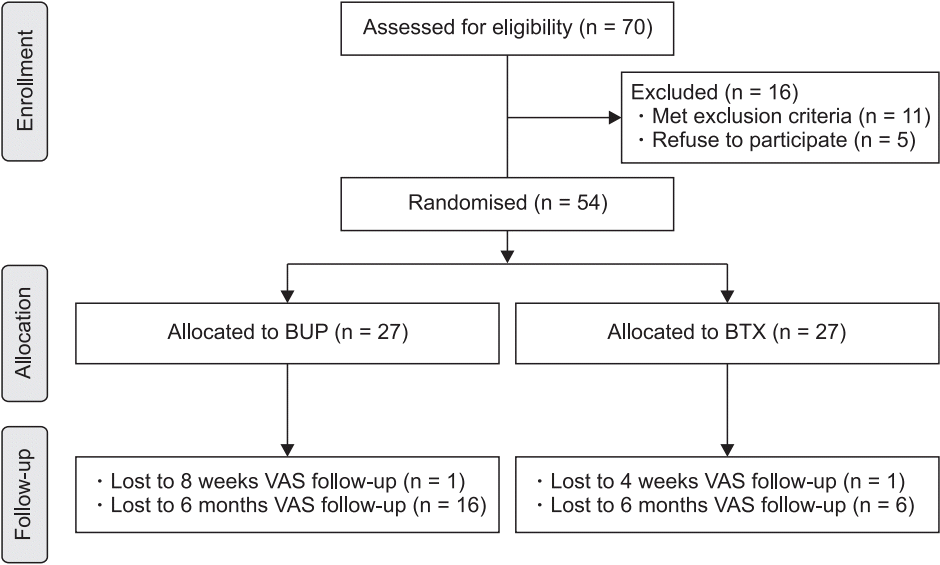
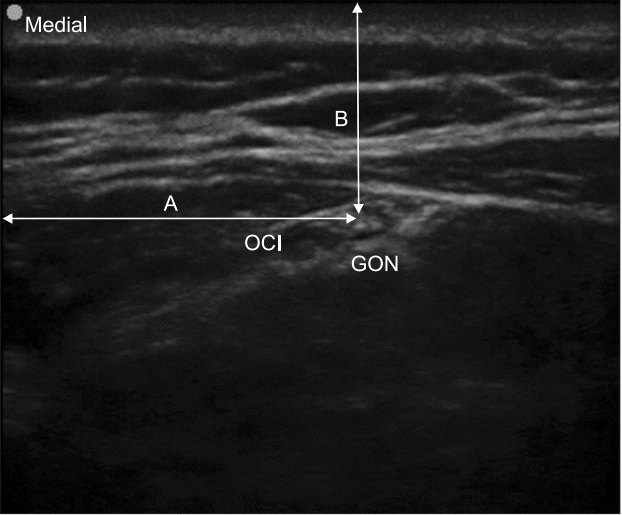
 XML Download
XML Download