Abstract
Background
Liver transplantation usually requires blood transfusion, and a red blood cell (RBC) antibody screen is essential for the prevention of a hemolytic reaction. Since proper ABO-compatible grafts are lacking, ABO-incompatible living donor liver transplantation (ABO-i LDLT) with desensitization is a feasible therapy. Desensitization includes intravenous rituximab injection and plasmapheresis before surgery.
Case
A 60-year-old female was diagnosed with hepatitis B virus-related hepatocellular carcinoma and planned for ABO-i LDLT. She tested positive in a RBC antibody screen over two years; however, she tested negative for the test after desensitization. Clinicians noted the seroconversion during induction, and thus, a delay in the preparation of adequate packed RBC was unavoidable.
Go to : 
Patients with chronic liver disease often have impaired coagulation and thrombocytopenia. They also have hyperdynamic circulation and the liver is well-known for receiving approximately a quarter of cardiac output. Liver transplantation (LT) is the treatment of choice for patients with end-stage liver disease. There is a risk of massive bleeding during LT and transfusion is frequently required. ABO typing and a red blood cell (RBC) antibody screen help to find safe blood and prevent a hemolytic transfusion reaction during allogeneic transfusion. ABO typing is also important for donor selection to prevent graft rejection. Since there are shortage with ABO-compatible graft, ABO-incompatible graft is a potential solution with comparable results [1,2].
Although there is no definite protocol for ABO-incompatible living donor liver transplantation (ABO-i LDLT), the recipient is usually treated with the anti-CD20 monoclonal antibody, rituximab and plasmapheresis before LT [1–3]. Rituximab suppresses B lymphocytes similar to that in a chemical splenectomy. Plasmapheresis removes antibodies in the plasma by exchanging fluid. These preparations are aimed to reduce antibodies against the incompatible ABO blood type antigens of the graft and to increase graft survival. Here we report a case of RBC antibody screen conversion before ABO-i LDLT.
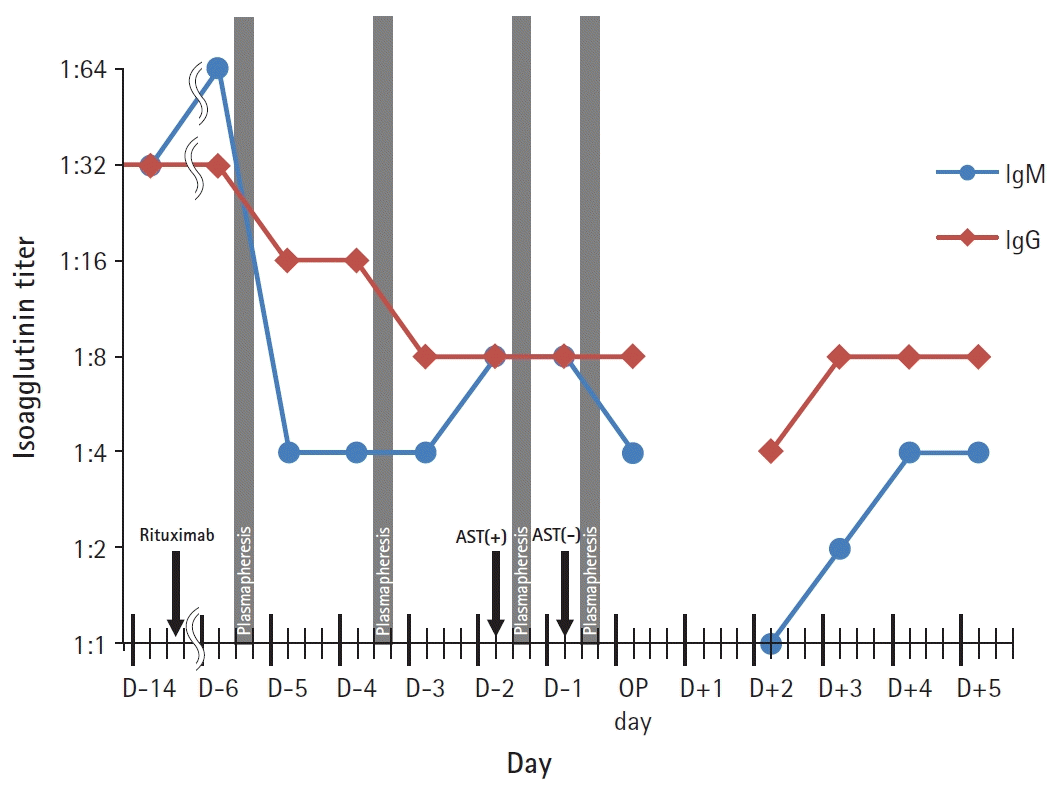
A 60-year-old female patient (height: 157 cm, body weight: 45.9 kg) was diagnosed with hepatitis B virus-induced liver cirrhosis and hepatocellular carcinoma (HCC) six years ago. She had been treated five times with trans-arterial chemoembolization and palliative radiotherapy. She used an albuterol inhaler once a month for asthma but never visited the emergency department with exacerbation. Two and four years prior to presentation, she had an umbilical hernia repair and total thyroidectomy for papillary thyroid carcinoma, respectively. She was taking an antiviral agent for hepatitis B virus, warfarin for portal vein thrombosis, and diuretics for ascites. Despite the hospitalization and medication, ascites was refractory and laboratory values had improved slightly. As her liver cirrhosis progressed, and HCC was unresectable, LT was planned. Preoperative hematocrit was 0.283% (normal range : 0.318–0.438%), hemoglobin was 9.2 g/dl (normal range: 11.2–14.8 g/dl), platelet count was 63000 /μl (normal range: 138000–347000 /μl), prothrombin time with an international normalized ratio was 1.17 (normal range: 0.90–1.10), sodium was 133 mmol/L (normal range: 135–145 mmol/L), and the model for end-stage liver disease score was 15 points. Vital signs were within the normal range. Preoperative chest radiography confirmed no active lung lesion, but the diaphragm was elevated toward the left side. The pulmonary function test showed a combined severe obstructive and moderate restrictive pattern. Transthoracic echocardiography showed diastolic dysfunction grade 1. The esophagogastroduodenoscopy revealed esophageal varices, portal hypertensive gastropathy, and gastric varices at cardia. One of her sons was willing to donate his liver, however, his blood type was AB while the patient’s was A. For immunosuppression, she received a single intravenous dose of rituximab (525 mg: 375 mg/m2 body surface area) two weeks prior to LT. Isoagglutinin immunoglobulin M (IgM) and G (IgG) titers against B antigen were measured before the rituximab injection and every morning for 7 days before the surgery, using a standard direct-agglutination technique (Fig. 1). Our hospital protocol is based on the American Society for Apheresis and the American Association of Blood Banks 2016 guidelines for apheresis [4]. Target isoagglutinin titer was less than 1 : 16, and 13 units of AB type fresh frozen plasma (FFP) as 1 estimated plasma volume (EPV) were used for each plasmapheresis (2 h). Target isoagglutinin titer was achieved by two consecutive plasmaphereses and LT was scheduled for the next day after two more plasmaphereses. The latest RBC antibody screen 1 days prior to operation was negative so it did not draw the clinicians’ attention. In the operation theater, electrocardiography, pulse oximetry, and non-invasive blood pressure were conducted. Two puffs of albuterol were administered. We asked the blood bank to prepare 5 units of packed RBCs of blood group A, considering the patient’s medical history, operation history, and laboratory evaluations. Anesthesia was induced with thiopental sodium (250 mg), rocuronium (50 mg), and 5 volume% of sevoflurane following preoxygenation for 3 min. After intubation, radial artery cannulation was performed on the right side with a 20 gauge (G) catheter and bispectral index (BIS, Medtronic, USA) monitoring was started. However, the hospital blood bank informed us that her RBC antibody screen test had been positive 2 days prior to surgery. We reviewed her medical record thoroughly and found that a pack of single-donor platelets (SDP) had been transfused twice, 3 and 2 years prior to presentation. Prior to the first transfusion, she was negative for RBC antibody screen. However, prior to the second transfusion, she was positive for the antibody screen and both anti-C (Rh system) and anti-M (MNS system) were identified [5]. The blood bank also noticed that there was only one unit of matched packed RBCs. The induction was postponed for 3 h until four more units of packed RBCs from other blood banks were received and cross-matched. Two 20 G catheters (SAC-00820 20 G 8
cm, Arrow International, USA) were inserted in the right femoral artery and vein. A 7 French central catheter (REF CS-15703-E 7 Fr 3 lumen 20 cm, Arrow International, USA) was placed through the left internal jugular vein under ultrasound guidance. She already had an 8.5 French permanent catheter inserted through the right internal jugular vein as a route for plasmapheresis. The magnetic induction fluid warmer (Belmont Instrument, Fluid Management system 2000, USA) was connected and the FloTrac/Vigileo system (Edwards Lifesciences, USA) monitoring started. Due to the stricture in the inferior vena cava (IVC), the surgeon performed IVC reconstruction and re-perfused three times. Methylprednisolone (500 mg) was infused during portal vein anastomosis and basiliximab (20 mg) was infused after reperfusion for immunosuppression. After the bleeding around the hepatic artery was controlled, the diaphragm was repaired, and two chest tubes were inserted. The patient was transferred to the surgical intensive care unit with an open abdomen. The total anesthesia time was 16 h and 30 min and the patient received approximately 17,500 ml of crystalloid, 1,200 ml of 5% albumin, 1,500 ml of 6% hydroxyethyl starch (Volulyte, Fresenius Kabi, Germany), 5 units of pre-storage leukocyte-reduced RBCs, 5 units of leukocyte-depleted RBCs, 4,872 ml of Cell Saver (Haemonetics, USA) blood, 9 units of blood type AB FFP, 2 units of blood type AB SDP, and 12 units of blood type AB cryoprecipitate. Intraoperative blood loss expressed with lost red cell mass was 4,123 ml [6] and urine output was 1,320 ml. The wound was closed in the operation theater on postoperative day (POD) 2 and wedge biopsy of the transplanted liver was also conducted. The biopsy revealed centrilobular hemorrhagic necrosis of hepatocytes, implying outflow impairment. The graft was not functioning well with stricture of the hepatic vein. As mechanical ventilation was prolonged, tracheostomy was applied on POD 7. Both IgM and IgG antibodies gradually increased but stayed under the target range (1 : 4 and 1 : 8, respectively) (Fig. 1). No further plasmapheresis was performed as the low level of IgM titer was maintained. Re-transplantation had been planned while the patient waited in the intensive care unit; however, she expired due to sepsis on POD 31.
Written informed consent for publication could not be obtained from the deceased patient.
Go to : 
There are 346 RBC antigens and 308 of them are assigned to 36 blood group systems. These blood group systems can be classified based on carbohydrates, glycophorins, complement regulation, adhesion and receptor molecules, transporters and channels, and enzymes [7]. Generally, blood typing for the ABO and D antigens and RBC antibody screen are performed before major surgery. The RBC antibody screen is known as an antibody screen test. It can be performed manually or by automation and by mixing the recipient’s plasma and two or three collections of clinically significant RBC antigens. If the screen shows any agglutination, it is considered positive, and an antibody identification test is required to detect the specific antibody responsible. The compatibility test or cross-matching is required before RBC transfusion. Matching only for blood typing has a 99.8% chance of compatible transfusion. The possibility can be increased to 99.95% with RBC antibody screen and cross-matching [8]. Unlike the ABO antibody, other RBC antibodies are rarely produced spontaneously, and such antibodies are called unexpected antibodies. In this case, the patient developed the RBC antibody after platelet transfusion without pregnancy or RBC transfusion. Platelets express a few RBC antigens that rarely generate antibodies, and there can be residuals or fragments of RBCs remaining in the platelet concentrates [5,9].
There was only a 3% chance of having proper packed RBCs since the frequency of C-negative and M-negative phenotypes in the Korean population are about 13% and 25%, respectively [10]. As liver transplantation (LT) usually requires blood transfusion, we routinely prepare intraoperative cell salvage to reduce allogenic transfusion. Autotransfusion can reduce immunologic events and contains 2,3-diphosphoglycerate (2,3-DPG). In stored RBCs, 2,3-DPG levels are decreased, and the oxyhemoglobin dissociation curve shifts left. Nevertheless, time is required until sufficient blood can be collected. In this case, because of the patient’s cancer, the blood needed to be filtered to remove possible cancer cells [11].
As the use of ABO-incompatible organs is increasing, immunosuppressive therapy is important to prevent both hyperacute rejection and acute antibody-mediated rejection (AMR) [1,2]. B lymphocytes produce antibodies including RBC antibodies, so their suppression during ABO-incompatible organ transplantation is imperative. Rituximab targets the CD20 antigen on the surface of B lymphocytes and depletes them. Morimoto et al. [12] found B lymphocytes were not detectable after two weeks of rituximab injection and the effect lasts for several months [12] and that rituximab injection earlier than 7 days before transplantation significantly reduced the frequency of AMR in ABO-i LDLT [13]. Standardizing the time of administration and the dosage adjustment still require further investigation [1,2,13].
Perioperative plasmapheresis reduces anti-A or anti-B antibody titers and improves graft survival in ABO-incompatible organ transplantation. There is no standard recommendation for target antibody titer. A titer of 1 : 64 or above would increase the risk of AMR and complications of transplantation. Plasmapheresis can be performed with FFP, albumin, or crystalloids. Volume exchange with 1 EPV removes 63% of antibodies, while 1.4 EPV removes 75%. For most cases, a volume of 1–1.4 EPV is exchanged [4]. In our case, levels of IgM and IgG against B antigen titers were reduced or equal a day after plasmapheresis (Fig. 1). Levels of IgM against B antigen doubled 2 days after the second plasmapheresis. After plasmapheresis, intravascular re-equilibration and re-synthesis can increase antibody titer [14]. After the third plasmapheresis, the RBC antibody screen converted from positive to negative. There was no agglutination with the machine and the laboratory performed another manual RBC antibody screen, but it was still negative. Although plasmapheresis aims to remove a specific antibody, large molecular-weight substances including other antibodies, complement components, and albumin can be removed [15]. In our case, serial plasmapheresis might have removed not only antibodies against B antigen but also anti-M and anti-C. As the number of antibodies is reduced by plasmapheresis, the concentration is also reduced by dilution. Rituximab has a role in sustained undetected RBC antibody screens by depleting memory B lymphocytes. Antibodies cannot be adequately replenished by the depleted B lymphocytes.
In our electronic medical record system, if a patient is positive for an RBC antibody screen once, the patient is always marked with ‘Ab’ in the patient information window. On the other hand, the RBC antibody screen result window displays only for the specific test. We overlooked the patient information window and only focused on the latest result window because the RBC antibody screen rarely changes from positive to negative. A system revision for the RBC antibody screen result window will improve patient management and safety. In conclusion, meticulous concern on the serial results of RBC antibody screens is necessary after immunosuppression, before ABO-incompatible solid organ transplantation.
Go to : 
Notes
Author Contributions
Eun kyung lee (Writing – original draft)
Gaab soo Kim (Writing – review & editing)
Insun Song (Investigation)
Go to : 
References
1. Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008; 47:143–52.

2. Kim JM, Kwon CH, Joh JW, Han SB, Sinn DH, Choi GS, et al. Case-matched comparison of ABO-incompatible and ABO-compatible living donor liver transplantation. Br J Surg. 2016; 103:276–83.

3. Kim JM, Kwon CHD, Joh JW, Han S, Yoo J, Kim K, et al. ABO-incompatible living donor liver transplantation with rituximab and total plasma exchange does not increase hepatocellular carcinoma recurrence. Transplantation. 2018; 102:1695–701.

4. Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American society for apheresis: the seventh special issue. J Clin Apher. 2016; 31:149–62.

5. Reckhaus J, Jutzi M, Fontana S, Bacher VU, Vogt M, Daslakis M, et al. Platelet transfusion induces alloimmunization to D and non-D Rhesus antigens. Transfus Med Hemother. 2018; 45:167–72.

6. Bang SR, Ahn HJ, Kim GS, Yang M, Gwak MS, Ko JS, et al. Predictors of high intraoperative blood loss derived by simple and objective method in adult living donor liver transplantation. Transplant Proc. 2010; 42:4148–50.

7. Lane WJ, Westhoff CM, Uy JM, Aguad M, Smeland-Wagman R, Kaufman RM, et al. Comprehensive red blood cell and platelet antigen prediction from whole genome sequencing: proof of principle. Transfusion. 2016; 56:743–54.

9. Garraud O, Cognasse F, Moncharmont P. Immunological features in the process of blood platelet-induced alloimmunisation, with a focus on platelet component transfusion. Diseases. 2019; 7:7.

10. Hong YJ, Chung Y, Hwang SM, Park JS, Kwon JR, Choi YS, et al. Genotyping of 22 blood group antigen polymorphisms and establishing a national recipient registry in the Korean population. Ann Hematol. 2016; 95:985–91.

11. Han S, Kim G, Ko JS, Sinn DH, Yang JD, Joh JW, et al. Safety of the use of blood salvage and autotransfusion during liver transplantation for hepatocellular carcinoma. Ann Surg. 2016; 264:339–43.

12. Morimoto H, Ide K, Tanaka Y, Ishiyama K, Ohira M, Tahara H, et al. Different sensitivity of rituximab-treatment to B-cells between ABO-incompatible kidney and liver transplantation. Hum Immunol. 2016; 77:456–63.

13. Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014; 14:102–14.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download