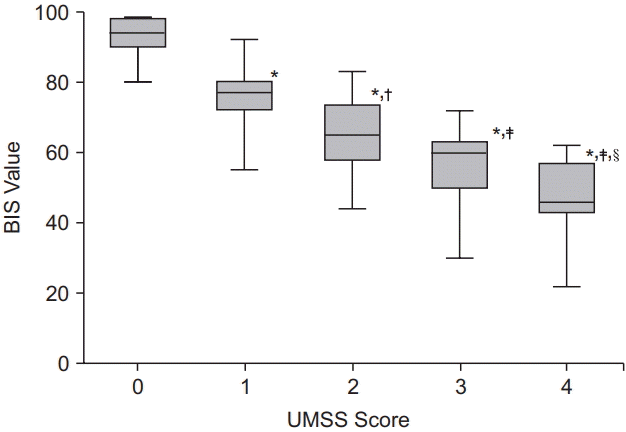
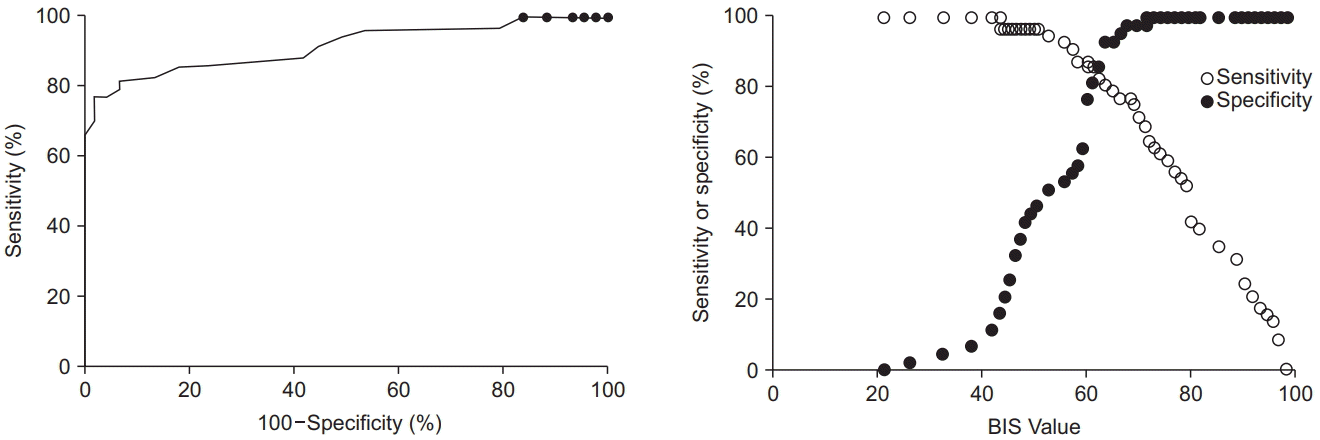
1. Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K, Naughton N. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS). Br J Anaesth. 2002; 88:241–5.

2. Rousseau AF, Ledoux D, Sabourdin N, Richard P, Damas P, Constant I. Clinical sedation and bispectral index in burn children receiving gamma-hydroxybutyrate. Paediatr Anaesth. 2012; 22:799–804.

3. Liu J, Singh H, White PF. Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesth Analg. 1997; 84:185–9.

4. Singh H. Bispectral index (BIS) monitoring during propofol-induced sedation and anaesthesia. Eur J Anaesthesiol. 1999; 16:31–6.

5. Choudhry DK, Brenn BR. Bispectral index monitoring: a comparison between normal children and children with quadriplegic cerebral palsy. Anesth Analg. 2002; 95:1582–5.

6. Yılbaş AA, Ayhan B, Akıncı SB, Sarıcaoğlu F, Aypar Ü. The effect of different end-tidal desflurane concentrations on bispectral Index values in normal children and children with cerebral palsy. Turk J Anaesthesiol Reanim. 2013; 41:200–5.
7. Saricaoglu F, Celebi N, Celik M, Aypar U. The evaluation of propofol dosage for anesthesia induction in children with cerebral palsy with bispectral index (BIS) monitoring. Paediatr Anaesth. 2005; 15:1048–52.

8. Costa VV, Torres RV, Arci EC, Saraiva RA. Comparison of the bispectral index in awake patients with cerebral palsy. Rev Bras Anestesiol. 2007; 57:382–90.
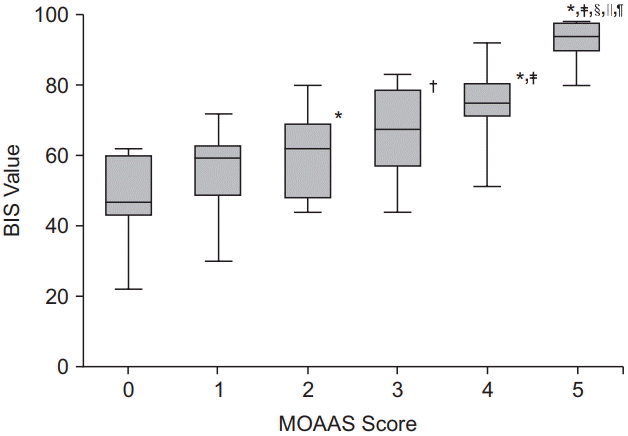
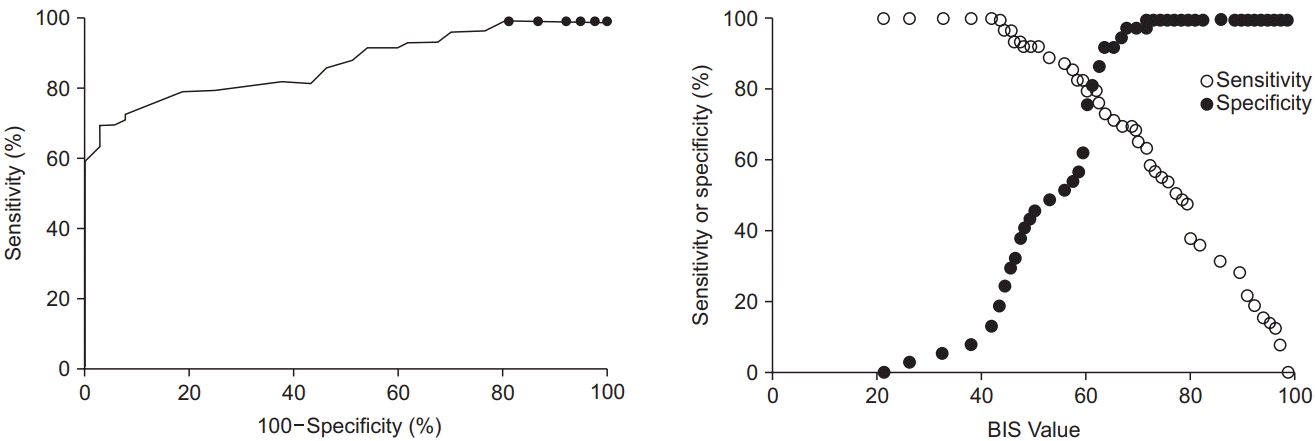
9. Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/ Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990; 10:244–51.
10. Malviya S, Voepel-Lewis T, Tait AR. A comparison of observational and objective measures to differentiate depth of sedation in children from birth to 18 years of age. Anesth Analg. 2006; 102:389–94.

11. Sciusco A, Standing JF, Sheng Y, Raimondo P, Cinnella G, Dambrosio M. Effect of age on the performance of bispectral and entropy indices during sevoflurane pediatric anesthesia: a pharmacometric study. Paediatr Anaesth. 2017; 27:399–408.

12. Powers KS, Nazarian EB, Tapyrik SA, Kohli SM, Yin H, van der Jagt EW, et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics. 2005; 115:1666–74.

13. Sadhasivam S, Ganesh A, Robison A, Kaye R, Watcha MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. 2006; 102:383–8.

14. Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004; 43:247–55.

15. Kim NY, Lee IO, Lim BG, Kim HZ, Kong MH, Lee MK, et al. Comparison of bispectral index (BIS) and entropy in patients with cerebral palsy during sevoflurane induction. Korean J Anesthesiol. 2009; 57:422–7.

16. McDermott NB, VanSickle T, Motas D, Friesen RH. Validation of the bispectral index monitor during conscious and deep sedation in children. Anesth Analg. 2003; 97:39–43.

17. Malviya S, Voepel-Lewis T, Tait AR, Watcha MF, Sadhasivam S, Friesen RH. Effect of age and sedative agent on the accuracy of bispectral index in detecting depth of sedation in children. Pediatrics. 2007; 120:e461–70.

18. Guignard B, Menigaux C, Dupont X, Fletcher D, Chauvin M. The effect of remifentanil on the bispectral index change and hemodynamic responses after orotracheal intubation. Anesth Analg. 2000; 90:161–7.

19. Reeves ST, Havidich JE, Tobin DP. Conscious sedation of children with propofol is anything but conscious. Pediatrics. 2004; 114:e74–6.

20. Moerman AT, Herregods LL, De Vos MM, Mortier EP, Struys MM. Manual versus target-controlled infusion remifentanil administration in spontaneously breathing patients. Anesth Analg. 2009; 108:828–34.

21. Nieuwenhuijs DJ, Olofsen E, Romberg RR, Sarton E, Ward D, Engbers F, et al. Response surface modeling of remifentanil-propofol interaction on cardiorespiratory control and bispectral index. Anesthesiology. 2003; 98:312–22.

22. Watcha MF. Investigations of the bispectral index monitor in pediatric anesthesia: first things first. Anesth Analg. 2001; 92:805–7.





 Citation
Citation Print
Print



 XML Download
XML Download