Abstract
During laparoscopic surgery, carbon dioxide (CO2) pneumothorax can develop due to a congenital defect in the diaphragm. We present a case of a spontaneous massive left-sided pneumothorax that occurred during laparoscopy-assisted gastrectomy, because of an escape of intraperitoneal CO2 gas, under pressure, into the pleural cavity through a congenital defect in the esophageal hiatus of the left diaphragm. This was confirmed on intraoperative chest radiography and laparoscopic inspection. This CO2 pneumothorax caused tolerable hemodynamic and respiratory consequences, and was rapidly reversible after release of the pneumoperitoneum. Thus, a conservative approach was adopted, and the remainder of the surgery was completed, laparoscopically. Due to the high solubility of CO2 gas and the extra-pulmonary mechanism, CO2 pneumothorax with otherwise hemodynamically stable conditions can be managed by conservative modalities, avoiding unnecessary chest tube insertion or conversion to an open procedure.
Although pneumothorax can occur at any time during laparoscopic surgery, a specific type of pneumothorax occurs subsequent to carbon dioxide (CO2) pneumoperitoneum creation. This CO2 pneumothorax, although rare, is caused by escape of the intraperitoneal CO2 gas, under pressure, into the pleural cavity through a congenital defect in the diaphragm [12].
In cases of major pneumothorax, due to underlying lung diseases, immediate simple aspiration or chest tube drainage is generally recommended [3]. However, most cases of CO2 pneumothorax, although large, can be managed successfully by conservative treatments unless it does not have a tension physiology or there is severe hemodynamic compromise [2456]. This may be due to the high solubility of CO2 gas (therefore, rapid resolution after release of the pneumoperitoneum) and the extra-pulmonary mechanism (therefore, no damage to the lung parenchyma) [4]. In such cases, unnecessary chest tube drainage during surgery will cause loss of pneumoperitoneum through draining, with attendant complications [34].
To have the capacity to treat this complication conservatively, anesthesiologists should be aware of the nature of CO2 pneumothorax. Thus, we describe a case of a massive left-sided CO2 pneumothorax that occurred during a laparoscopy-assisted gastrectomy and that was managed conservatively without the conversion to an open procedure.
A 65-year-old female (149 cm, 52 kg) was undergoing an elective laparoscopy-assisted Billroth-I gastrectomy for early gastric cancer at the distal antrum. She had no significant medical history except for undergoing uterine myomectomy under general anesthesia 16 years earlier. Results of routine laboratory tests, chest radiograph, and electrocardiogram were all normal.
Without premedication, anesthesia was induced with propofol (120 mg) and rocuronium (50 mg), and was maintained with sevoflurane in air and 50% oxygen with volume-controlled mechanical ventilation (tidal volume: 450 ml, respiration rate: 12 /min, inspiration/expiration ratio: 1/2). Neuromuscular block was maintained with vecuronium.
A laparoscopy-assisted gastrectomy was performed using a five-trocar technique. Abdominal insufflation was performed with CO2 gas, and the trocars were placed uneventfully. CO2 pneumoperitoneum was maintained at an insufflation pressure of 12 mmHg. The patient was placed in a 15–20° reverse Trendelenburg position to assist with surgical exposure.
At 15 min after the beginning of CO2 insufflation, saturation of peripheral oxygen (SpO2) decreased to 94%, and the peak airway pressure increased gradually from 16 to 37 cmH2O. As the inspired fraction of oxygen (FIO2) was immediately increased from 50% to 100%, the SpO2 increased to 97%. Chest auscultation revealed normal breath sounds over the right chest but greatly reduced breath sounds on the left. Presuming that this could be due to right endobronchial migration of the tracheal tube due to pneumoperitoneum, the tube was pulled out 1.5 cm. However, there were no improvements in the airway pressure (peak airway pressure of 28 cmH2O despite a tidal volume reduced to 400 ml) and in air entry on the left-side chest. The patient remained stable hemodynamically with a blood pressure of 105–135/60–90 mmHg and a heart rate of 70–90 beats/min, while the arterial blood gas analysis at this time (FIO2: 1.0, end-tidal CO2 pressure: 47 mmHg) showed a pH and partial pressures of arterial CO2 and oxygen of 7.25, 56.9 mmHg, and 89.3 mmHg, respectively.
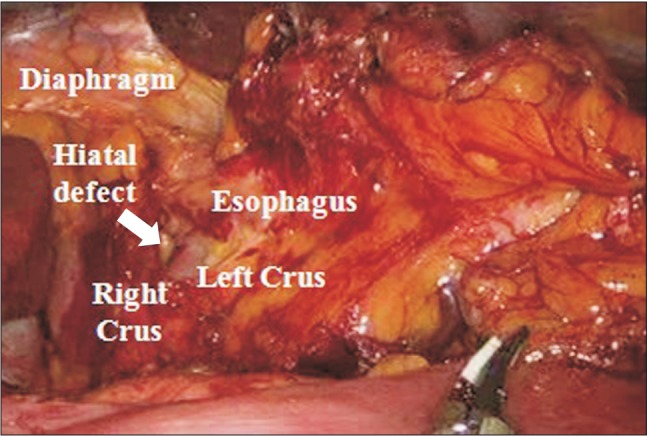
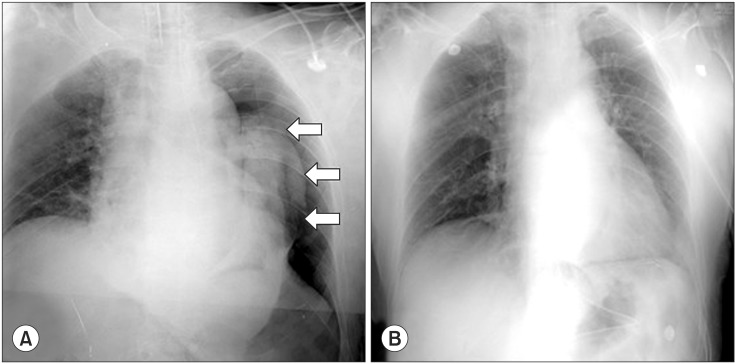
A fiber-optic bronchoscope examination was performed to rule out endobronchial intubation and bronchial obstruction due to secretions. As a result, the tracheal tube was found to be correctly positioned, and both main-stem bronchi were clear of obstructions. A left-sided pneumothorax was then suspected, and the surgeon was asked to inspect the left hemi-diaphragm with the laparoscope. The surgeon discovered a small defect in the esophageal hiatus of the left diaphragm (Fig. 1). A subsequent chest radiograph confirmed the presence of a massive left-sided pneumothorax (Fig. 2A). No subcutaneous emphysema was detected.
As the abdomen was deflated for the portable chest radiography, normal breath sounds over the left chest were auscultated, and the peak airway pressure decreased to 20 cmH2O. Thus, the cause of the pneumothorax was diagnosed as escape of the intraperitoneal CO2 gas, under pressure, into the pleural cavity through a congenital defect in the diaphragm. Because this CO2 pneumothorax caused few hemodynamic and respiratory consequences and could be rapidly reversed after the release of pneumoperitoneum, the surgery was continued laparoscopically at the same insufflation pressure (12 mmHg). The revised ventilator parameters, including positive end-expiratory pressure (PEEP), were FIO2: 0.7, tidal volume: 450 ml, respiration rate: 14 /min, inspiration/expiration ratio: 1/2, and PEEP: 5 cmH2O. Additionally, the surgeon was requested to finish the procedure under pneumoperitoneum as soon as possible. As pneumoperitoneum was re-established, her breath sounds was diminished gradually in the left chest, but the peak airway pressure and SpO2 remained stable, at 21 cmH2O and 100%, respectively. During the remainder of the operation, the patient was stable hemodynamically. At 45 min after the resumption of pneumoperitoneum, the surgery was completed uneventfully. After manual deflation of the abdomen, an alveolar recruitment maneuver (manual inflation of 40 cmH2O for 15 s) was performed several times [7]. After confirming that bilateral equal breath sounds had returned, the patient was extubated and transported to the postanesthesia care unit (PACU).
A further chest radiograph in the PACU revealed complete resolution of the left-sided pneumothorax (Fig. 2B). Her postoperative course was uneventful, and she was discharged on the eighth postoperative day with no further problem.
Although rare, pneumothorax has been described as a complication
of laparoscopic surgery in up to 2% of patients [8]. The potential causes of intraoperative pneumothorax can be divided into two categories: congenital defects and iatrogenic. The iatrogenic causes are multiple including rupture of emphysematous bullae or bleb due to barotrauma, direct injury to the diaphragm during dissection, central line placement or even trocar sites. During CO2 pneumoperitoneum, pneumothorax may also be caused by congenital defects in the diaphragm or other defects around sites where the aorta, vena cava, and esophagus traverse the diaphragm [12].
This type of intraoperative CO2 pneumothorax is an extremely rare complication in patients with no prior symptoms of gastroesophageal reflux disease. It has occasionally been reported mainly in laparoscopic cholecystectomy [291011]. To date, only one case has been reported to occur during a laparoscopic gastrectomy [12]. CO2 pneumothorax is more likely to occur on the right side during laparoscopic cholecystectomy whereas when performing surgery around the esophagus, it occurred mostly on the left side. This may be due to the differences in surgical exposure of the omentum which naturally covers a diaphragmatic defect or weak point. In our case, a hiatal defect was demonstrated by observation of the left diaphragm with the laparoscope, whereas the cause of pneumothorax was uncertain in the previously reported case that occurred during laparoscopic gastrectomy [12].
When considering that the diaphragm is normally covered with the liver and omentum, some degree of association between surgical positions and the development of CO2 pneumothorax can be suspected. Head-up position plus CO2 pneumoperitoneum may increase the incidence and severity of CO2 pneumothorax by pushing the liver and omentum downward, thereby leading to exposure of the diaphragmatic defect in the pressurized abdominal cavity. On the other hand, a head-down position may have a protective effect against the development of CO2 pneumothorax. For this reason, this type of pneumothorax may have been reported mainly in laparoscopic upper abdominal surgical procedures.
The three most consistent signs are abrupt and marked increases in the end-tidal CO2 pressure and airway pressure, and a decrease in the SpO2 [1]. In our case, endobronchial intubation was initially suspected because several clinical presentations (an increase in the peak airway pressure, a decrease in SpO2, and loss of breath sounds on the left chest) had developed after the creation of pneumoperitoneum. However, subsequent bronchoscopic examination excluded this and the possibility of left main bronchial obstruction due to secretions.
Although pneumothorax was then suspected, the patient's cardiorespiratory status was sufficiently stable to wait for chest radiography before proceeding with chest tube insertion. As a result, a massive left-sided pneumothorax was confirmed by intraoperative chest radiography. Because normal breath sounds on left chest had returned rapidly after the discontinuation of pneumoperitoneum, we could diagnose a pressure-limited CO2 pneumothorax with an extra-pulmonary origin. In such a condition, a surgical tear or congenital defect in the diaphragm is the most likely point for the escape of CO2 gas into the thoracic cavity. In our case, the possibility of an inadvertent surgical tear could be excluded, because no surgical dissection had been performed around the diaphragm in the development of the pneumothorax. Thus, we considered the possibility of a congenital defect in the diaphragm, which was then demonstrated by the surgeon's laparoscopic examination.
The diaphragm forms from the fusion of four embryonic components, and improper development or fusion of these structures can result in congenital weak points or defects in the diaphragm. Stretching and expansion of these weak points by the elevated pressure of the pneumoperitoneum may result in pneumothorax [2456].
When faced with a patient with CO2 pneumothorax, treatment should be proportional to the hemodynamic and respiratory compromise [245]. If a tension or bilateral CO2 pneumothorax is suspected during a laparoscopic surgery, the appropriate action is an immediate release of the pneumoperitoneum and decompressive CO2 aspiration or chest tube drainage. Fortunately, tension pneumothorax did not occur in this case. One possible reason for this is that the communication between the pleural and the peritoneal cavities did not function as a check valve. Additionally, positive pressure ventilation and the high solubility of CO2 gas in blood might both be 'favorable' factors [456].
In most cases of CO2 pneumothorax, as rapid recovery invariably occurs after the release of pneumoperitoneum, the operation can be continued laparoscopically with a suitable degree of lung collapse [24]. In these situations, the main goal of the supportive therapy is to decrease the pressure gradient between the abdominal and pleural cavities.
From a surgeon's perspective, it is necessary to reduce the intra-abdominal pressure, shorten the pneumoperitoneum time, or level the patient position for continuing the laparoscopic surgery [2413]. Additionally, surgeons should plug the diaphragmatic defect with the help of the omentum at the end of the operation [13].
From an anesthesiologist's perspective, nitrous oxide should be turned off, and ventilator strategy should be changed. Desaturation and CO2 retention are usually alleviated by increases in FIO2 and minute ventilation. Specifically, keeping an adequate level of inspiratory pressure can decrease the pressure gradient between the abdominal and pleural cavities during inspiration, while it decreases the incidence of additional pleural damage and reduces the negative inotropic effect of intermittent positive pressure ventilation [2]. The addition of PEEP (if possible, equivalent to intra-abdominal pressure) can minimize the collection of the intrapleural CO2 gas not only during inspiration but also during expiration [56]. Additionally, gradual reexpanding the collapsed lung should be achieved before extubation by adopting one of various alveolar recruitment maneuvers [14].
Among the management modalities mentioned, lower abdominal insufflation pressure was not used in our case. We thought that shortening of the pneumoperitoneum time (i.e., facilitating the surgical procedure) might be more beneficial for reducing the overall CO2 inflow to the pleural cavity than the application of lower abdominal insufflation pressure.
In summary, this case suggests that pneumothorax can occur during laparoscopic surgery due to the escape of intraperitoneal CO2 gas, under pressure, into the pleural cavity through a congenital defect in the diaphragm. Unlike the pneumothorax secondary to lung trauma, most CO2 pneumothorax cases are well tolerated and rapidly reversed after the release of pneumoperitoneum. Thus, a conservative approach is a priority for the treatment of CO2 pneumothorax in an otherwise hemodynamically stable patient, avoiding unnecessary chest tube insertion or conversion to an open procedure. Of course, it is essential to keep close communication and collaboration with the surgeon(s) and to monitor the patient's hemodynamic and respiratory conditions vigilantly throughout the pneumoperitoneum.
References
1. Wahba RW, Tessler MJ, Kleiman SJ. Acute ventilatory complications during laparoscopic upper abdominal surgery. Can J Anaesth. 1996; 43:77–83. PMID: 8665641.

2. Karayiannakis AJ, Anagnostoulis S, Michailidis K, Vogiatzaki T, Polychronidis A, Simopoulos C. Spontaneous resolution of massive right-sided pneumothorax occurring during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005; 15:100–103. PMID: 15821624.

3. Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax. 2003; 58(Suppl 2):ii39–ii52. PMID: 12728149.

4. Phillips S, Falk GL. Surgical tension pneumothorax during laparoscopic repair of massive hiatus hernia: a different situation requiring different management. Anaesth Intensive Care. 2011; 39:1120–1123. PMID: 22165368.

5. Joris JL, Chiche JD, Lamy ML. Pneumothorax during laparoscopic fundoplication: diagnosis and treatment with positive end-expiratory pressure. Anesth Analg. 1995; 81:993–1000. PMID: 7486090.
6. Moore M, O'Brien K. Carbon dioxide pneumothorax treatment with positive end-expiratory pressure. Anaesthesia. 2004; 59:622–623. PMID: 15144316.

7. Claxton BA, Morgan P, McKeague H, Mulpur A, Berridge J. Alveolar recruitment strategy improves arterial oxygenation after cardiopulmonary bypass. Anaesthesia. 2003; 58:111–116. PMID: 12562405.

8. Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000; 95:704–709. PMID: 10775733.

9. Gabbott DA, Dunkley AB, Roberts FL. Carbon dioxide pneumothorax occurring during laparoscopic cholecystectomy. Anaesthesia. 1992; 47:587–588. PMID: 1385681.

10. Moore M, O'Brien K. Carbon dioxide pneumothorax treatment with positive end-expiratory pressure. Anaesthesia. 2004; 59:622–623. PMID: 15144316.

11. Prystowsky JB, Jericho BG, Epstein HM. Spontaneous bilateral pneumothorax: complication of laparoscopic cholecystectomy. Surgery. 1993; 114:988–992. PMID: 8236025.
12. Cha SM, Jung YH, Kim DS, Kang H, Baek CW, Koo GH. Spontaneous pneumothorax during laparoscopy-assisted Billroth-I gastrectomy -A case report-. Korean J Anesthesiol. 2010; 58:405–408. PMID: 20508801.

13. Singhal T, Balakrishnan S, Hussain A, Grandy-Smith S, Paix A, El-Hasani S. Management of complications after laparoscopic Nissen's fundoplication: a surgeon's perspective. Ann Surg Innov Res. 2009; 3:1. PMID: 19193220.

14. Kim JK. Importance of alveolar recruitment strategy revisited. Korean J Anesthesiol. 2014; 67:75–76. PMID: 25237441.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download