Abstract
A 6-year-old boy was scheduled for thoracic magnetic resonance imaging under deep sedation with midazolam 1.8 mg and propofol 100 µg/kg/min via intravenous injection. He showed emergence delirium in the post-anesthesia care unit. The staff attempted to calm him by administering flumazenil as an antidote for midazolam, propofol for further sedation, and meperidine. However, this was not successful. A psychiatrist recommended the use of antipsychotics. Administration of risperidone led to immediate resolution of the boy's symptoms and relaxed him. The use of antipsychotic drugs is not common for anesthesiologists, but should be considered for treating uncontrolled emergence delirium after anesthesia.
Emergence delirium (ED) is defined as "a cluster of disturbing behaviors that can occur in the early post-anesthetic period" [1], and is a very common pediatric disturbance with an incidence ranging from 10% to 80% [23]. ED is characterized by non-purposeful restlessness, agitation, crying, disorientation, and paranoid ideation [123]. The Pediatric Anesthesia Emergence Delirium (PAED) scale, which scores ED based on eye contact, purposeful actions, awareness, restlessness, and consolability, has been validated to evaluate the incidence and severity of ED [3].
ED is a major cause of pediatric postoperative distress and can increase the workload of post-anesthesia care unit (PACU) staff members [1]. If pediatric patients show ED-like behaviors in the PACU, the staff will usually administer sedative or opioid agents to calm them [14]. However, many of the clinical symptoms of ED are similar to those of paradoxical midazolam reactions [256]. Therefore, flumazenil has also been used to treat postoperative ED associated with midazolam exposure [2]. In this case study, the patient experienced uncontrolled ED even after clinicians used classical treatments, such as sedative drugs, opioids, and flumazenil. The patient was finally calmed after administration of risperidone, an antipsychotic agent. Therefore, we suggest this alternative treatment option for uncontrolled ED after sedation.
A 6-year-old boy (26 kg/108 cm) was scheduled for a pre- and post-contrast magnetic resonance imaging (MRI) of the thorax. He had received chemotherapy followed by surgical resection for ganglioneuroblastoma in the left posterior mediastinum. He had made a complete recovery from the cancer in 2012. From 2012 to 2016, MRI was performed regularly due to a remnant nodule in the T6 vertebral body. He underwent MRI five times using midazolam 1–2 mg and propofol 100–300 mg (20–50 µg/kg/min), and no problems occurred during sedation. Vital signs before sedation were normal, and a 24-G intravenous catheter was inserted into the patient's right forearm. During the exam, blood pressure, electrocardiography, and oxygen saturation were measured and remained stable. Supplemental oxygen was administered via a face mask, 1.8 mg of midazolam and 20 mg of 2% lidocaine were injected, and anesthesia was maintained with propofol 100 µg/kg/min. No other medication was given. During the uneventful 58-minute MRI scan, 100 ml of IV lactated Ringer's solution and 210 mg of propofol were infused (100 µg/kg/min).
The patient was transported to the PACU, initially sleeping calmly with normal vital signs. An oxygen face mask was applied, and 5 L/min of oxygen was routinely administered. After 20 minutes, the patient awoke and attempted to remove the oxygen mask. He talked with his parents and made eye contact. However, 10 minutes after awakening, the patient attempted to leave the bed and to violently remove the monitors and the intravenous catheter. His parents gently restrained him because they were unable to communicate with him. He was very anxious, unresponsive to his parents' attempts to console him, and restless. His heart rate increased to 141 beats/min and respiratory rate increased to 60 breaths/min. However, his oxygen saturation level remained 99% without the oxygen supply. His PAED score was 14 (makes eye contact with caregiver = 2, actions are purposeful = 1, aware of surroundings = 3, restless = 4, inconsolable = 4), corresponding to a diagnosis of ED. Another intravenous catheter was inserted, and flumazenil (0.1 mg) and propofol (20 mg) were administered intravenously, but the patient's restlessness remained. Meperidine 12.5 mg and propofol 10 mg were administered, and the patient was sedated. After 10 minutes, he awoke and again showed mild restlessness and hyperactivity. He said that he felt stifled in the PACU, and his oxygen saturation remained over 97%. Thus, we decided to remove the oxygen mask and allowed him to sit in a chair outside the PACU, and we contacted a psychiatrist. We continued talking with the patient, continuously checking his oxygen saturation levels. He became less hyperactive once he was outside the PACU, and after approximately 10 minutes, he was able to respond to verbal commands by nodding his head. However, he remained restless throughout his stay at the hospital, and his condition did not meet the post-MRI outpatient discharge criteria. Due to the patient's delirium, the psychiatrist examined him carefully, diagnosed him with pediatric ED, and prescribed risperidone (Rispedal®, Janssen Korea, Seoul, Korea) 0.5 mg by oral administration. The patient's restlessness disappeared 30 minutes after drug administration, and he was again able to engage in conversation. We closely monitored him in the PACU, and 40 minutes later, he was discharged home without any other symptoms of delirium.
Three months later, we conducted a telephone interview with the child and his mother. He had no history of migraines or other headaches, and no history of nightmares. He did not remember anything about his restlessness, agitation, crying, disorientation, and hyperactivity in the PACU. There is no specific family history related to anesthesia, delirium, or any other psychiatric disturbance.
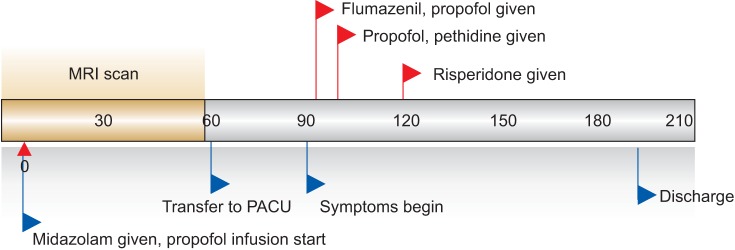
Our patient had already received sedation for MRI examinations 11 times before without experiencing any side effects, including ED. However, this time he demonstrated ED, which did not respond to flumazenil, propofol, and meperidine. We consulted a psychiatrist, and the patient's symptoms were controlled with antipsychotic drugs approximately 60 minutes after he received midazolam and propofol (Fig. 1).
The etiology of ED is still unknown but is thought to be multifactorial and to include postoperative pain and the pharmacokinetics and pharmacodynamics of anesthetic agents [4]. Many ED risk factors, such as the child's age, ethnicity, socioeconomic background, temperament, previous hospital experience, anxiety level, and the length and type of procedure, have been reported in previous studies [14789]. However, the findings of those studies contradicted the results of others [49]. Our patient had very few ED risk factors; he had not undergone any painful ear, eye, or dental procedures and he did not have a previous history of ED or preoperative anxiety. He only had two risk factors: young age (6 years) and rapid onset of ED (10 minutes).
After the patient's diagnosis of ED, we tried to calm him, but he became violent and potentially could have hurt himself. Therefore, we attempted to manage him pharmacologically, first by administering 0.1 mg of flumazenil intravenously. Voepel-Lewis et al. [10] theorized that paradoxical benzodiazepine reactions could be an underlying factor contributing to the onset of ED. Flumazenil, a competitive antagonist at the benzodiazepine-binding site of the gamma-aminobutyric acid type A receptor, has been used to successfully treat postoperative ED associated with midazolam exposure [610]. In our case, we observed the patient for 5 minutes, but administration of flumazenil had no effect. After that, we used propofol 20 mg intravenously. Many articles have recommended intravenous boluses of sedative agents, such as midazolam 0.025–0.1 mg/kg or propofol 0.5–1.0 mg/kg, as a pharmacological 'rescue' treatment for ED [49]. While we chose propofol, dexmedetomidine (0.3 mg/kg) has been recommended as a preventive treatment against ED and has been found to be more effective than propofol in recent research [11]. We did not use dexmedetomidine because we had already used two types of sedatives; moreover, it would take a long time for the dexmedetomidine to take effect after its infusion. Lastly, we administered meperidine 12.5 mg intravenously. Cravero et al. [12] reported that the addition of a small dose of fentanyl to sevoflurane anesthesia decreases the incidence of emergence agitation independent of the pain-control effects. Thus, opioids could help manage ED, even for non-painful procedures, even though the time required to meet the hospital discharge criteria was unchanged by the addition of this small dose of fentanyl in that research. Our patient fell asleep, but 10 minutes later, his hyperactivity returned. However, after the psychiatrist administered risperidone, the patient remained calm and became cooperative.
Antipsychotic drugs are not familiar to anesthesiologists. However, from a psychiatrist's point of view, children are thought to be more vulnerable to delirium because of immature and evolving structural and biochemical brain development [78]. Thus, psychiatrists have chosen neuroleptic drugs (haloperidol, risperidone) for first-line pharmacological treatment of delirium in children [78]. Clinical experience with haloperidol in pediatric delirium supports its beneficial effects, although that is based purely on uncontrolled case reports [8]. Risperidone is a strong antagonist of 5HT, D2, and adrenergic α1 and α2 receptors, is a mild histaminergic H1 antagonist, and has little effect at the cholinergic receptor sites [13]. It is available as an oral tablet and is comparable to IM haloperidol with and without concomitant lorazepam [14]. Its effect begins 30 to 60 minutes after administration and its half-life is 20–22 hours; once-daily dosing is sufficient [14]. Orthostatic hypotension is a common initial side effect, and extrapyramidal symptoms can occur in a dose-dependent manner. However, risperidone has lower rates of extrapyramidal symptoms and sedation than haloperidol [13]. Risperidone was used in this case study because compared to risperidone, haloperidol is associated more strongly with cardiac dysrhythmias, such as QT interval prolongation, and extrapyramidal side effects, such as dystonia, tardive dyskinesia, akathisia, and neuroleptic malignant syndrome [815]. These concerns, coupled with risperidone's fewer short-term side effects, have most likely contributed to prescribers' preference for and increased use of risperidone in the treatment of children and adolescents in general. However, if a patient is not cooperative with oral medication administration and shows severe symptoms, intravenous haloperidol injection is recommended.
In conclusion, when ED is not controlled via classic treatments, such as the use of midazolam, propofol, and opioids, the administration of antipsychotic drugs, such as haloperidol and risperidone, should be considered for calming the patient.
References
1. Rosen HD, Mervitz D, Cravero JP. Pediatric emergence delirium: Canadian pediatric anesthesiologists' experience. Paediatr Anaesth. 2016; 26:207–212. PMID: 26559766.

2. Drobish JK, Kelz MB, DiPuppo PM, Cook-Sather SD. Emergence delirium with transient associative agnosia and expressive aphasia reversed by flumazenil in a pediatric patient. A A Case Rep. 2015; 4:148–150. PMID: 26035220.

3. Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004; 100:1138–1145. PMID: 15114210.

4. Dahmani S, Delivet H, Hilly J. Emergence delirium in children: an update. Curr Opin Anaesthesiol. 2014; 27:309–315. PMID: 24784918.
5. Saltik IN, Ozen H. Role of flumazenil for paradoxical reaction to midazolam during endoscopic procedures in children. Am J Gastroenterol. 2000; 95:3011–3012. PMID: 11051408.
6. Sanders JC. Flumazenil reverses a paradoxical reaction to intravenous midazolam in a child with uneventful prior exposure to midazolam. Paediatr Anaesth. 2003; 13:369–370. PMID: 12753459.

7. Rey JM. Psychiatry and pediatrics. Geneva: IACAPAP 2015;2015. p. 11–12.
8. Williams DT. Delirium and catatonia. Lewis's Child and Adolescent Psychiatry. 4th ed. Martin A, Volkmar FR, editors. Philadelphia: Lippincott Williams & Wilkins;2007. p. 648–650.
9. Wong DD, Bailey CR. Emergence delirium in children. Anaesthesia. 2015; 70:383–387. PMID: 25764401.

10. Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003; 96:1625–1630. PMID: 12760985.

11. Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth. 2013; 7:296–300. PMID: 24015133.

12. Cravero JP, Beach M, Thyr B, Whalen K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth Analg. 2003; 97:364–367. PMID: 12873918.

13. Pollock BG, Semla TP, Forsyth CE. Psychoactive drug therapy. In : Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard's Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill;2009. p. 767–778.
14. Garris S, Hughes C. Acute agitation. In : Tintinalli JE, Stapczynski J, Ma O, Yealy DM, Meckler GD, Cline DM, editors. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York: McGraw-Hill;2016. p. 1952–1957.
15. Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet. 1999; 37:435–456. PMID: 10628896.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download