Abstract
Metastatic spinal tumors are usually quite difficult to treat. In patients with metastatic spinal tumors, conventional radiotherapy fails to relieve pain in 20–30% of cases and open surgery often causes considerable trauma and complications, which delays treatment of the primary disease. Percutaneous vertebroplasty (PVP) is considered to be useful in achieving rapid pain control and preventing further vertebral collapse due to spinal metastasis. However, symptoms of intraspinal neural compression can be contraindications to PVP. To overcome this problem, we performed PVP following targeted bipolar radiofrequency decompression, and examined the effect of the combined treatment in relieving severe radicular pain related to spinal cord compression caused by malignant metastatic tumors.
Metastatic spinal tumors develop in an estimated 5–10% of all cancer patients and the vertebral spine is the most common site of painful skeletal metastases [12]. The treatment of metastatic spinal tumors is difficult. The goals of treatment are to obtain pain relief, to stabilize the vertebrae, and to improve patient's quality of life. In general, conventional radiotherapy is the treatment of choice, but it fails to relieve pain in 20–30% of patients, as well as causing damage to surrounding structures, particularly in the case of tumors located close to nerves and plexuses [3]. Conventional surgical resection often results in direct injuries to nerves, as well as additional complications. Furthermore, it is not suitable for the treatment of patients with multiple spinal metastases or those with immunodeficiencies or short life expectancies [4].
To avoid these limitations, a minimally invasive procedure [5] and percutaneous vertebroplasty (PVP) have been developed to treat vertebral body metastatic lesions. The first use of PVP was reported in patients with vertebral hemangiomas [6]. Since then, PVP has been used as an effective treatment option for patients with intractable pain related to osteoporotic vertebral compression fractures and spinal metastases [789]. The performance of PVP is now considered useful to achieve prompt pain control and prevent further vertebral collapse and spinal cord compression in patients with vertebral metastases [10]. However, symptoms of neural compression more severe than the axial pain have been deemed relative contraindications to PVP, because the procedure can result in progressive neurologic deficits and severe pain due to any remaining compression [78].
To overcome these problems, we designed a treatment whereby PVP was combined with targeted bipolar radiofrequency decompression (BRFD), which was used to shrink the tumor mass as well as induce coagulation to prevent bleeding from the tumor.
A 51-year-old man who had been diagnosed with sigmoid colon cancer four years earlier had undergone treatment with a subtotal colectomy, chemotherapy, and radiotherapy. He had received a craniotomy to excise a brain tumor one month previously. His height was 177 cm and his weight was 73 kg. His current chief complaint was a two-month history of severe back pain, accompanied by a tingling sensation, with radiation of the pain into both inguinal regions, especially the right. The patient's knee jerk reflexes were normal without neurologic deficits. Magnetic resonance imaging (MRI) revealed multiple metastatic lesions in the patient's lumbar spine, at L1, L2, L4, and L5. The main cause of the patient's symptoms was a lesion in L1, which had metastasized to the posterior vertebral body wall and right pedicle, resulting in spinal cord compression (Fig. 1). The patient had received radiation therapy before visiting the pain clinic, and he was taking oxycodone 40 mg and gabapentin 300 mg three times a day with no complications. Despite these interventions, he continued to experience significant pain. The pain was aggravated by changes in position from lying to sitting and relieved when he was lying. He rated his pain as 6–10 on the numeric 0–10 rating scale (NRS).The patient's average pain score with respect to the NRS was 8/10. However, he did not wish to undergo open surgery because of his poor general condition and short life expectancy. We initially planned to reduce the patient's pain using an epidural block prior to secondary interventional management, but he was not able to tolerate the prone position required to receive the epidural block due to the severe radiating pain he experienced in that position. Therefore, it was necessary to treat the patient's pain maximally in a single therapeutic session. Thus, we proposed to perform BRFD with PVP and the patient agreed to undergo the procedure.
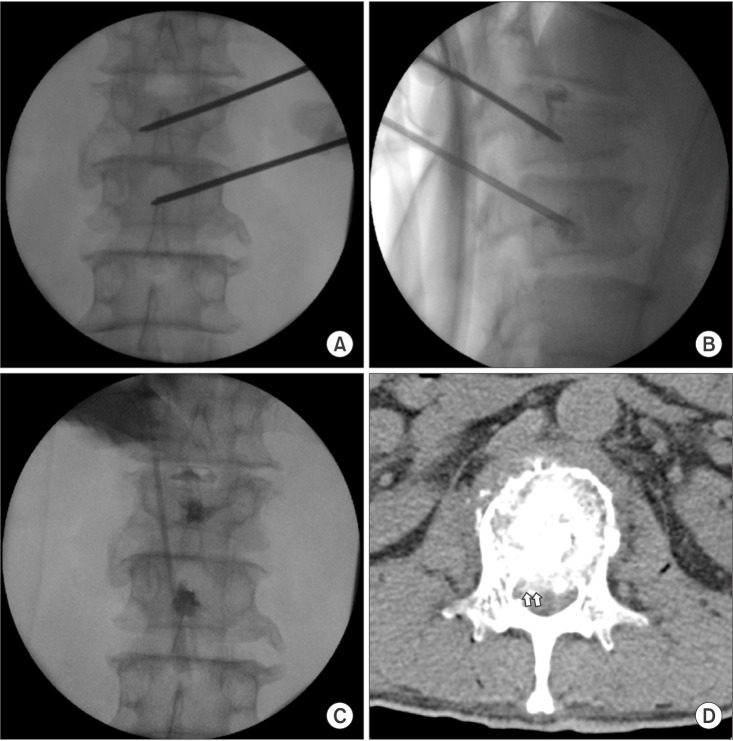
We proceeded under conscious sedation administered by an experienced anesthesiologist, because of the patient's poor general condition and in order to prevent neurological complications during the procedure. For analgesia, a 12 µg fentanyl patch was applied to the patient's right upper thorax twelve hours before the procedure, and an intramuscular injection of pethidine 25 mg was also administered ten minutes before the procedure. One hour before the procedure, the patient received an intramuscular injection of midazolam 3 mg for sedation. During the procedure, the patient's heart rate, blood pressure, oxygen saturation, and electrocardiogram were monitored. The patient was placed on an operating table in the prone position with a flexed hip. His skin was prepared and draped in a sterile fashion. The entry point of the needle through the skin was determined using an axial MRI (Fig. 1B). The needle was inserted 9 cm lateral to the midline. After a subcutaneous 1% lidocaine injection, a small incision was made with a blade, and under continuous fluoroscopic monitoring, an 18 gauge needle and guidewire were inserted sequentially through the incision site until the tip of the needle reached the middle portion of the L1 pedicle. Then, a dilator was used to insert a 2.7 mm diameter cannula (Disc-FX® System, Elliquence, LLC, Baldwin, NY, USA) to the middle portion of the pedicle in the lateral view and the medial pedicular margin in the anteroposterior (AP) view. A trephine was inserted through the working channel to the posterior body line in the lateral view and the median vertebral line in the AP view. After removing the guidewire and trephine, a 2.5 mm diameter bipolar probe (Trigger-Flex® Bipolar System, Elliquence) was inserted through the working channel (Fig. 2). An energy generator (Surgi-Max®, Elliquence) that emits high radiofrequency and low temperature radiowaves was connected to the Trigger-Flex® Bipolar probe and we shrank the tumor mass by ablation using the turbo-modulation mode. We also used the bipolar hemo-modulation mode for thermocoagulation to prevent bleeding. We conducted five cycles of the procedure while subtracting from the midline of the vertebral body to the lateral border of the right pedicle at the L1 level. The procedure was repeated using the turbo-modulation mode and the hemo-modulation mode in shifts per five second. Once the radiofrequency ablation procedure was completed, we performed PVP at the L1 and L2 bodies. The bone puncture needles were inserted into the posterior 1/3 aspect of the L1 body and the medial part of the L2 body in the lateral view and the median vertebral line in the AP view through the right pedicle. Two ml of sterile polymethyl methacrylate (PMMA) powder (Exolent Spine, Elmdown, Ltd., London, UK) and the liquid components were injected to the L1 and L2 bodies under frequent fluoroscopic lateral and AP views to avoid PMMA leakage into the venous system and the epidural space (Figs. 3A–3C). We completed the procedure after confirming via fluoroscope that there was no leakage of cement. The patient's radiating pain disappeared after the procedure, and there were no complications. Following the procedure, the patient's vital signs were monitored and he was observed for any neurological complications for 30 min in the outpatient recovery room, after which he was transferred to the general ward.
The patient was discharged the day after the procedure because he reported relief of his pain to a level of 1/10 on the NRS without any complications, and he was able to sit without pain. He was then able to undergo continuous chemotherapy and radiotherapy for treatment of the multiple bone metastases. At a 6-month follow-up, the patient's average pain score with respect to the NRS was 1/10. He could sit and walk in his daily life without the recurrence of radicular pain.
The incidence of spinal metastasis tends to increase as the life expectancy of cancer patients increases, due to early detection techniques and improvements in medical and surgical treatment [2]. Treatments for cancer patients with spinal metastases include radiation therapy, surgery, or a combination of the two. However, conventional surgical resection is not an option in patients with short life expectancies or for the treatment of multiple metastatic spinal lesions due to its long recovery period and high morbidity and mortality [11]. Thus, radiotherapy alone is in common used, but pain relief may be delayed or incomplete following radiation therapy for spinal metastases [4]. Therefore, alternative treatment methods, such as vertebroplasty and kyphoplasty, are currently considered for these patients [912]. PVP is a minimally invasive procedure that not only destroys tumor cells but also has an internal stabilization effect [3]; thus, pain control is achieved more rapidly and further vertebral collapses are prevented [10]. Even though the procedure is simple and rapid, the clinical usage of PVP in malignant vertebral fractures with symptoms of neural compression has been rare because the patient's symptoms can be aggravated by the remaining neural compression and by the leakage of PMMA from the epidural space [41314]. Therefore, in this case, we attempted an alternative approach; we performed PVP followed by BRFD in a patient with an intraspinal canal tumor mass.
Compared to PVP alone, the combination of BRFD with PVP has several advantages. The size of the metastatic mass compressing the spinal cord could be reduced by BRFD. The ablation effects of the radiofrequency and the exothermic reaction of the PMMA produce a synergic effect resulting in a more complete destruction of sprouting nerve endings, which drastically reduced the back pain of the patient in this case. After bipolar radiofrequency heat ablation had decreased the size of the tumor, we could apply more bone cement due to the increased size of the vertebral body cavity. Therefore, we could expect to restore vertebral height and stability and improve the safety of the procedure by preventing infiltration of the tumor into the spinal canal.
If we performed the procedure in patients with metastatic bone tumors by conventional radiofrequency treatment, it might be difficult to control bleeding from tumors themselves as well as from other potential structures, since the conventional radiofrequency equipment does not usually include a system for bleeding control. However, there are differences between existing radiofrequency ablation equipment and the bipolar radiofrequency system used in this study. We used the Disc-FX® System, with which bleeding control was possible in the hemomodulation mode. The bipolar hemo-modulation mode hardened the surrounding tissue, preventing the potential spread of bone cement to the posterior body wall, epidural space, and veins. The absorption of the surgical energy source of the bipolar radiofrequency (Surgi-Max®, Elliquence) showed a depth of penetration of 0.02 mm with high-frequency, low-temperature radio energy, below 42℃, so the possibility of damage to neural tissues near the target was low [15]. On the other hand, the holmium: YAG laser has a 0.4 mm depth of penetration. Based on these data, the bipolar radiofrequency instrument (Surgi-Max®, Elliquence) can be considered safer than the holmium: YAG laser.
Even though our method has many advantages, it also has limitations. Several authors have performed interventional tumor removal using nucleus rongeurs [14]. Therefore, we attempted to use forceps to reduce the tumor in the current case. However, in this case, as pulsatile bleeding was observed through the working channel in the field, it was not safe to utilize forceps for debulking the intraspinal canal metastatic tumor. Moreover, the use of forceps was also dangerous as the fluoroscope showed only an indirect image. If we had employed a smaller-sized endoscope, the decompression could have been safer and more effective because we could have observed the intraspinal canal tumor mass directly through the endoscope.
In conclusion, we recommend the use of PVP and BFRD to treat intraspinal metastases in patients with intractable radicular pain who do not respond to conservative treatment or who cannot undergo open surgery, if a proper trajectory of approach can be obtained during preoperative imaging. Further clinical trials to determine the long-term effectiveness of the combined procedure should be conducted in the future.
References
3. Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Atwell TD, Farrell MA, et al. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol. 2006; 35:1–15. PMID: 16205922.

4. Shimony JS, Gilula LA, Zeller AJ, Brown DB. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 2004; 232:846–853. PMID: 15273339.

5. Joo YC, Ok WK, Baik SH, Kim HJ, Kwon OS, Kim KH. Removal of a vertebral metastatic tumor compressing the spinal nerve roots via a single-port, transforaminal, endoscopic approach under monitored anesthesia care. Pain Physician. 2012; 15:297–302. PMID: 22828683.
6. Ofluoglu O. Minimally invasive management of spinal metastases. Orthop Clin North Am. 2009; 40:155–168. viiiPMID: 19064063.

7. Kassamali RH, Ganeshan A, Hoey ET, Crowe PM, Douis H, Henderson J. Pain management in spinal metastases: the role of percutaneous vertebral augmentation. Ann Oncol. 2011; 22:782–786. PMID: 20966180.

8. Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010; 376:1085–1092. PMID: 20701962.

9. Woo JH, Park HS, Han JI, Kim DY. Vertebroplasty for the compression of the dorsal root ganglion due to spinal metastasis. Pain Physician. 2013; 16:E405–E410. PMID: 23877464.
10. Bhatt AD, Schuler JC, Boakye M, Woo SY. Current and emerging concepts in non-invasive and minimally invasive management of spine metastasis. Cancer Treat Rev. 2013; 39:142–152. PMID: 22959872.

11. Taylor JW, Schiff D. Metastatic epidural spinal cord compression. Semin Neurol. 2010; 30:245–253. PMID: 20577931.

12. Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg Br. 2010; 92:1054–1060. PMID: 20675746.

13. Saliou G, Kocheida el M, Lehmann P, Depriester C, Paradot G, Le Gars D, et al. Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement. Radiology. 2010; 254:882–890. PMID: 20177099.

14. Li Y, Gu YF, Sun ZK, Wu CG, Li YD, Wang W, et al. Comparison of percutaneous vertebroplasty with and without interventional tumour removal for malignant vertebral compression fractures with symptoms of neurological compression. Eur Radiol. 2013; 23:2754–2763. PMID: 23760302.

Fig. 1
Preoperative lumbar MRI. (A) T2-weighted sagittal MRI and (B) axial MRI showed the diffuse infiltration of the tumor invasion (white arrows) throughout the entire L1 vertebral body. It extended to the central canal and compressed the spinal cord. (B) The entry point was established 9 cm lateral to the midline using the axial MRI.
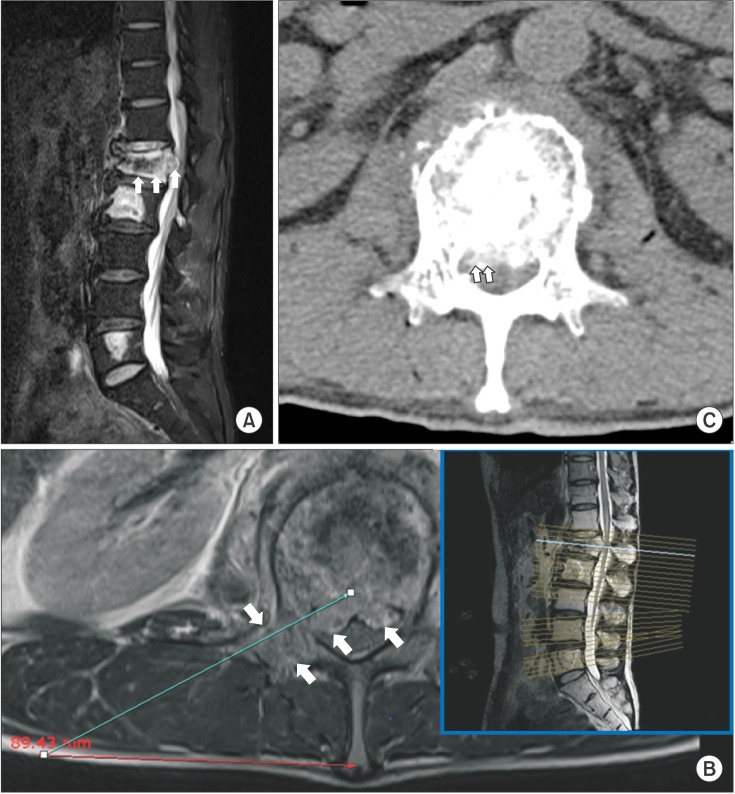
Fig. 2
Fluoroscopic images during bipolar radiofrequency decompression. (A) Bipolar probe was inserted through working cannula located in the middle portion of the pedicle in the lateral view, and (B) the medial side of the pedicle in the anteroposterior view.
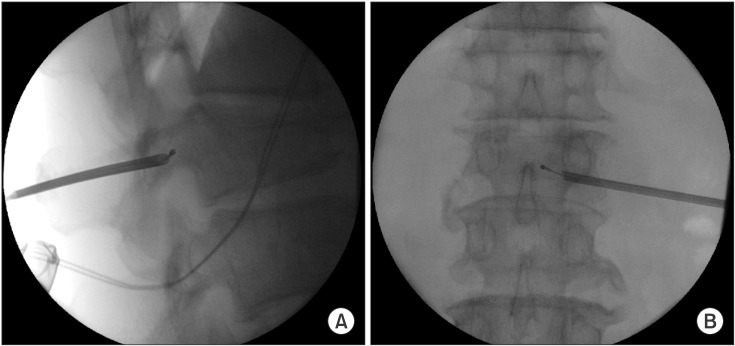
Fig. 3
Fluoroscopic images and CT images with vertebroplasty at L1 and L2. (B) Fluoroscopic images show that the puncture needles were inserted into the posterior 1/3 aspect of the L1 body and the medial part of the L2 body in the lateral view and (A) along the median vertebral line in the AP view through the right pedicle of each. (B, C) The small amount of PMMA injected spread from the middle to the posterior third part of each vertebral body, without spreading into the venous system or the epidural space. (D) The postoperative axial CT image showed the existence of a radiolucent mass (white arrows) in the spinal canal at the L1 level. Bone cement was injected at the precise location without cement leakage.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download