Abstract
Sodium nitroprusside (SNP) is an anti-hypertensive drug, commonly used to decrease the systemic vascular resistance and lower the blood pressure. When the amount of cyanide generated by the SNP exceeds the metabolic capacity for detoxification, cyanide toxicity occurs. Under general anesthesia and cardiopulmonary bypass (CPB), it may be difficult to detect the development of cyanide toxicity. In cardiac surgical patients, hemolysis, hypothermia and decreased organ perfusion, which emphasize the risk of cyanide toxicity, may develop as a consequence of CPB. In particular, hemolysis during CPB may cause an unexpected overproduction of cyanide due to free hemoglobin release. We experienced a patient who demonstrated SNP tachyphylaxis and cyanide toxicity during CPB, even though the total amount of SNP administered was much lower than the recommended dose. We therefore report this case with a review of the relevant literature.
Sodium nitroprusside (SNP) is a potent vasodilator, which acts on venous and arterial smooth muscle. It effectively decreases the systemic vascular resistance and lowers the blood pressure. Under normal circumstances, SNP converts to thiocyanate and is then excreted by the kidneys. However, if the amount of cyanide generated from the SNP exceeds the metabolic capacity for detoxification, cyanide toxicity occurs. The risk factors identified as possible etiologies for SNP-induced cyanide toxicity include renal dysfunction, prolonged infusion duration and/or high doses of SNP, drug interactions, and the young age of patient [1,2]. The risk of cyanide toxicity from SNP may be increased in cardiac surgical patients [3]. Studies have determined that SNP preferentially reacts on hemoglobin with sulfhydryl groups to release free cyanide [4]. Normally, the reaction between the SNP and hemoglobin is limited by the erythrocyte membrane that prevents the exposure of SNP to hemoglobin within the cells. However, hemolysis during cardiopulmonary bypass (CPB) accelerates the immediate release of free cyanide from SNP. We experienced a case of a patient who demonstrated nitroprusside tachyphylaxis and cyanide toxicity during CPB, and was treated with sodium thiosulfate. Below, we report the case with a review of the relevant literature.
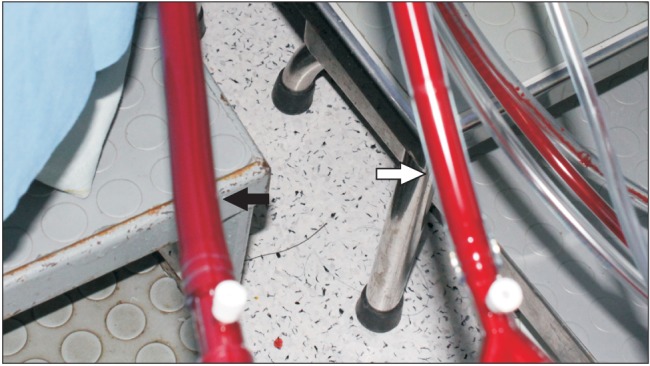
A 77-year-old woman of, 160 cm and 50 kg, underwent an ascending and hemiarch replacement surgery due to an aortic dissection and saccular aneurysm in the ascending aorta. She complained of chest and left upper quadrant pain which had intensified 3 days prior. She presented a history of hypertension on medication (β blocker, calcium channel blocker, diuretics, aspirin and clopidogrel). The results of the preoperative laboratory tests including a liver enzyme, serum creatinine and urine examination, were within the normal limits, except for the estimated glomerular filtration rate (eGFR), which was 51.19 ml/min/1.73 m2. The initial blood pressure in emergency room was 170/70 mmHg. The blood pressure was controlled with a labetalol infusion, and the systolic pressure was below 130 mmHg. SNP was not administered preoperatively. The heart rate was between 60 to 80 beats/min. The patient was premedicated with midazolam 1 mg and morphine 4 mg intramuscularly. Upon arrival in the operating room, the patient's vital signs showed a blood pressure of 145/65 mmHg, a heart rate of 45 beats/min, a respiration rate of 20 rates/min and a peripheral oxygen saturation of 97%. A pulmonary arterial catheter (Swan-Ganz CCOmbo CCO/SvO2; Edwards Lifesciences, Irvine, USA) was inserted through the right internal jugular vein before induction of the anesthesia. The anesthesia was induced without hemodynamic perturbation. The systolic blood pressure was maintained between 120 and 130 mmHg. Thirty minutes into the operation, the blood pressure had increased to 155/75 mmHg and the heart rate was 55 beats/min. An SNP infusion was initiated with 0.4 µg/kg/min. The response to the drug was prompt and the systemic blood pressure was easily maintained at 100/45 mmHg. The SNP infusion was discontinued 5 minutes later. For CPB priming, 2 units of packed RBC were transfused. The pump flow was 3.4 L/min. One hour after the cardiopulmonary bypass had started, an aortic cross clamp (ACC) was installed and a selective cerebral perfusion (SCP) was started. Hypothermia was induced with 27.7℃. The mean arterial pressure (MAP) was controlled at 70 mmHg for 45 minutes, but the MAP then increased to 100 mmHg. The SNP infusion was resumed with 0.4 µg/kg/min. During the next 15 minutes, the MAP rose to 120 mmHg and the dose of SNP was increased up to 2.4 µg/kg/min in order to lower the MAP. The SCP was stopped and the proximal ACC was taken off. The MAP continued to rise, reaching 140 mmHg, and the surgery was interrupted because of the bleeding at the suture site of the aorta. SNP tachyphylaxis was observed. Suspending cyanide toxicity, we discontinued the SNP infusion and administered labetalol continuously. The MAP started to decrease. At that point, bright red- colored blood similar to that of arterial line was observed at the venous line of the CPB (Fig. 1). Venous blood gas sampled from venous line of the CPB analyzed. As described in Table 1, the data of the venous blood gas analysis and MAP were serially altered. The mixed venous oxygen tension (PvO2) was 360.4 mmHg, the venous oxygen saturation (SvO2) was 99.8%, the pH was 7.56 and the lactate was 4.62 mmol/L. Venous blood from the pulmonary catheter was analyzed again. The PvO2 and SvO2 read 297.9 mmHg and 99.8% respectively. We started to infuse 12.5 g of sodium thiosulfate (ametox, DaiHan Pharm. Co., Ltd., Seoul, Korea) and the CPB was simultaneously weaned off. One hour later (after the CPB was stopped), another venous blood gas analysis was performed. The PvO2 and SvO2 had dropped to 24.9 mmHg and 50.2%, respectively. The pH was 7.47 and the lactate had decreased to 2.78 mmol/L. At that time, the blood pressure was 105/60 mmHg, the heart rate was 65 beats/min, the peripheral oxygen saturation 100% and the cardiac index 1.9 L/min/m2. The arterial blood pH was 7.34 and the partial pressure of the arterial oxygen (PaO2) was 430.4 mmHg. Because, aorta laceration had occurred at the end of the surgery, a second CPB was performed to repair the aorta. During the rest of the CPB, the MAP and other hemodynamic profiles were stably regulated. The total CPB time was 279 minutes and the aortic clamp time was 165 minutes. At the end of the operation, the SvO2 was 52%.
After 14 hours of anesthesia, the patient was taken to the intensive care unit (ICU) and her vital signs were as follows: blood pressure 117/64 mmHg, heart rate 75 beats/min with milrinone (0.75 µg/kg/min) and dobutamine (10 µg/kg/min). In the postoperative period, the patient showed signs of acute respiratory distress syndrome. The PaO2 was 64 mmHg at a fraction of the inspired oxygen 1.0 and other laboratory data were similar to the preoperative data.
The patient was sedated in the ICU with a sufentanil and propofol infusion. On the third post-operative day, left- side weakness was observed. A brain computed tomogram revealed an acute large infarcted area in the right middle cerebral artery territory and numerous small infarctions in the bilateral cerebral and cerebellar hemispheres. After 13 days of ICU stay, the patient expired from multiple organ failures.
SNP is one of the most popular antihypertensive drugs for aortic dissection. Each SNP molecule releases five cyanide molecules when broken down. The free cyanide molecule is transformed into thiocyanate by the rhodanase enzyme in the presence of thiosulfate. Both the cyanide and thiocyanate are renally eliminated. The clinical manifestations of cyanide toxicity are dizziness, oxygen extraction disorder, tachyphylaxis from SNP, coma, generalized seizure, and lactic acidosis [5]. However, under general anesthesia and CPB, some of these symptoms cannot be observed. During CPB and hypothermic circulatory arrest, the reduced liver and renal blood flow can emphasize the development of cyanide toxicity. Furthermore, an underlying chronic kidney disease inferring from a decreased preoperative eGFR might cause an accumulation of cyanide and thiocyanate. Moreover the rhodanase dysfunction induced by hypothermia, exaggerates the formation of free cyanide.
In this case, the patient's blood pressure showed a drop immediately after the SNP infusion. However, after the CPB started, it was considered that tachyphylaxis might rapidly develop even though the total administered dose of SNP was only 1.5 mg (0.03 mg/kg). This was the first observed sign of cyanide toxicity. Even though the pharmacological mechanisms of SNP tachyphylaxis have not yet been satisfactorily explained, it is known that a high level of blood cyanide interferes with vascular smooth muscle relaxation, and thus with vasodilation [6]. To minimize the risk of cyanide toxicity, some investigators have recommended the maximum total dose of SNP acutely administered not to exceed 1.0 mg/kg [7]. Several case reports of SNPinduced cyanide toxicity described patients having received a dose of SNP over 2 µg/kg/min and with infusion durations lasting longer than 24 hours [1]. In this case, the total amount of administered SNP (total amount: 1.5 mg, 0.03 mg/kg, maximum infusion rate: 2.4 µg/kg/min for 5 minutes) causing the manifestations of cyanide toxicity was found to be much lower than the recommended dose. The reasons for the manifestation of cyanide toxicity with dose lower than the clinically recommended dose cannot be clearly explained. Hemolysis as a consequence of CPB may explain, in part, the increased risk of cyanide toxicity that has been observed in cardiac surgical patients. Experiments have demonstrated that although SNP incubation with erythrocytes resulted in free cyanide release, the amount of free cyanide released increased of 5- to 8-fold when the SNP was incubated with human red blood cell lysates [4,8,9].
The second visible sign of cyanide toxicity in this patient was the similar blood color of the arterial and venous lines of the CPB (Fig. 1). Suspecting cyanide toxicity, PvO2 analysis of the blood from the CPB venous line and the pulmonary catheter line were performed. An extremely high PvO2 was found, indicating an oxygen extraction disorder. Following the administration of thiosulfate, the PvO2 gradually dropped. Similar results have been observed increased mixed venous blood O2 tension and decreased myocardial O2 consumption in a cyanide- intoxicated dog [10].
The patient did not present lactic acidosis, one of the characteristic manifestations of cyanide toxicity. Severe acidosis has not always been observed in patients with SNP complications, as shown by other case reports [11]. The early detection of cyanide toxicity before further progress of the metabolic acidosis might have been the reason. Another suggested explanation is that bicarbonate is commonly administered during CPB, preventing the observation of lactic acidosis.
The diagnostic test for cyanide toxicity is the serum cyanide level. The signs and symptoms of cyanide toxicity are difficult to confirm, and the use of a routine cyanide level check to prevent toxicity is not recommended [1]. Cautious use of SNP or modification of the therapy is required for patients presenting risk factors for developing toxicity (including renal or hepatic dysfunction, young age, and long term use). A prophylactic administration of thiosulfate is recommended in the case of a prolonged infusion of SNP [8]. During CPB, because of the rapid onset of the toxic effects from cyanide poisoning and the difficulty in performing a rapid sensitive analysis of the serum cyanide, prophylactic administration of thiosulfate may be needed.
The treatments for cyanide toxicity are hemodialysis, peritoneal dialysis and the administration of antidotes. Sodium thiosulfate (150 mg/kg or 12.5 g) increases the metabolism of cyanide. Sodium nitrite (5 mg/kg or 300 mg) oxidizes the hemoglobin into methemoglobin, which reacts with cyanide to form nontoxic cyanomethemoglobin. Hydrocobalamine also combines with cyanide to form cyanocobalamine [12,13].
In our center, an immediate serum cyanide level test was not available. Nevertheless, detection of the toxic symptoms and treatment were not delayed due to laboratory data confirmation in this case. Myocardial cyanide toxicity is not reversed in extremely high blood cyanide levels [10]. Therefore, empirical and preventive medication before further increasing the serum cyanide level may not be an irrational treatment [8,14].
Anesthesiologists should always remember that cyanide toxicity can readily occur during CPB, even with a much smaller dose of SNP than recommended. Even if the elevation of the serum cyanide level cannot confirm, in the presence of clinical manifestations and symptoms of cyanide toxicity, treatment should not be delayed.
References
1. Thomas C, Svehla L, Moffett BS. Sodium-nitroprusside-induced cyanide toxicity in pediatric patients. Expert Opin Drug Saf. 2009; 8:599–602. PMID: 19645589.
2. López-Herce J, Borrego R, Bustinza A, Carrillo A. Elevated carboxyhemoglobin associated with sodium nitroprusside treatment. Intensive Care Med. 2005; 31:1235–1238. PMID: 16041521.

3. Patel CB, Laboy V, Venus B, Mathru M, Wier D. Use of sodium nitroprusside in post-coronary bypass surgery. A plea for conservatism. Chest. 1986; 89:663–667. PMID: 3486098.
4. Smith RP, Kruszyna H. Nitroprusside produces cyanide poisoning via reaction with hemoglobin. J Pharmacol Exp Ther. 1974; 191:557–563. PMID: 4427294.
6. Posner MA, Rodkey FL, Tobey RE. Nitroprusside-induced cyanide poisoning: antidotal effect of hydroxocobalamin. Anesthesiology. 1976; 44:330–335. PMID: 1259191.
7. Michenfelder JD, Theye RA. Canine systemic and cerebral effects of hypotension induced by hemorrhage, trimethaphan, halothane, or nitroprusside. Anesthesiology. 1977; 46:188–195. PMID: 842872.

8. Marino PL, Sutin KM. Nonpharmaceutical toxidromes. The ICU Book. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2014. p. 985–986.
9. Cheung AT, Cruz-Shiavone GE, Meng QC, Pochettino A, Augoustides JA, Bavaria JE, et al. Cardiopulmonary bypass, hemolysis, and nitroprusside-induced cyanide production. Anesth Analg. 2007; 105:29–33. PMID: 17578949.

10. Tinker JH, Michenfelder JD. Cardiac cyanide toxicity induced by nitroprusside in the dog: potential for reversal. Anesthesiology. 1978; 49:109–116. PMID: 686414.
11. Cottrell JE, Casthely P, Brodie JD, Patel K, Klein A, Turndorf H. Mechanism and prevention of tachyphylaxis and cyanide toxicosis after nitroprusside-induced hypotension. Surg Forum. 1978; 29:308–310. PMID: 401173.
12. Butterworth JF, Mackey DC, Wasnick JD. Critical care. Morgan & Mikhail's Clinical Anesthesiology. 5th ed. Columbus: The McGraw-Hill Professional;2013. p. 1307–1308.
13. Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012; 24:225–238. PMID: 22672162.

14. Hall VA, Guest JM. Sodium nitroprusside-induced cyanide intoxication and prevention with sodium thiosulfate prophylaxis. Am J Crit Care. 1992; 1:19–25. PMID: 1307887.

Fig. 1
Bright red-colored blood is observed at the venous line of the cardiopulmonary bypass (black arrow) which is similar to that of the arterial line (white arrow).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download