Introduction
The major goal of postoperative pain management is to minimize the dose of medications, in order to reduce the side effects, while still providing adequate analgesia [
1]. Laparoscopic gynecologic surgery is less painful than open surgery. However, despite mild postoperative pain, many patients are still concerned about postoperative pain management. Postoperative pain management is a critical component of patient care and is associated with patient satisfaction. Intravenous patient-controlled analgesia (IV-PCA) with opioids and non-steroidal anti-inflammatory drugs (NSAIDs) is the most commonly utilized method for acute postoperative pain management. However, the administration of opioids has several adverse effects such as postoperative nausea and vomiting (PONV), dizziness, pruritus, and respiratory depression. The use of opioids for postoperative pain management is therefore avoided because almost 80% of patients experienced PONV following their use after laparoscopic gynecologic surgery [
2].
Nefopam has been used extensively in many European countries for the treatment of acute and chronic malignant and non-malignant pain. Previous studies have shown that nefopam 20 mg is equipotent to morphine 6–12 mg and meperidine 50 mg [
34]. In most studies, it has been administrated as an adjunctive drug in IV-PCA with opioids. Despite the analgesic efficacy of nefopam being equipotent to that of other opioids, no studies have supported the use of nefopam alone for IV-PCA.
The aim of this randomized, double-blind non-inferiority study was to evaluate the analgesic efficacy of IV-PCA using nefopam alone, compared with a combination of opioids and NSAIDs, after laparoscopic gynecologic surgery. We also compared the incidence rate of PONV, patient satisfaction with postoperative pain control, percentage of patients requiring additional opioids, and the incidence rate of postoperative adverse effects.
Materials and Methods
Enrolment
The study was approved by the Institutional Review Board and registered at the Clinical Research Information Service. After obtaining written informed consent, we enrolled 60 patients, with an American Society of Anesthesiologists (ASA) physical status of I–II, scheduled to undergo laparoscopic gynecologic surgery. Patients with pre-existing neurological and psychological deficits, hepatorenal dysfunctions, obesity (body mass index ≥ 30 kg/m2), addiction to opioids, hypersensitivity to NSAIDs, and those with a history of severe nausea and vomiting after IV-PCA, were excluded from the study.
Randomization
A list of random numbers generated by Microsoft Excel (Microsoft Co., Redmond, WA, USA) was used to randomize patients into two groups. The trial was carried out in a double-blind manner. The patients received either a combination of morphine 60 mg and ketorolac 180 mg (group A, n = 30), or nefopam 200 mg alone (group B, n = 30).
Intraoperative management
Laparoscopic gynecologic surgery under general anesthesia was performed on all patients. General anesthesia was induced with 5 mg/kg thiopental sodium and a single bolus of rocuronium 0.8 mg/kg was administered for tracheal intubation. Anesthesia was maintained using a 1.0 minimal alveolar concentration of desflurane with a fractional inspired oxygen concentration (FIO2) of 0.4, managed with the bispectral index (XP version 4.1; Aspect Medical Systems, Newton, MA, USA).
Patients were randomized into two groups. For postoperative pain management, group A (n = 30) received an IV-PCA pump (Gemstar; Hospira, Lake Forest, IL, USA) with 100 ml of normal saline mixed with morphine 60 mg and ketorolac 180 mg. Group B (n = 30) received an IV-PCA with 100 ml of normal saline mixed with nefopam 200 mg. In both groups, the basal infusion rate was set to 1 ml/h, the bolus dose was 1 ml, and the lockout time was 15 min. For PONV management, ramosetron 0.3 mg was routinely injected 30 min before the end of the surgery in both groups. Before skin closure, both groups received 8 ml of a loading dose via an IV-PCA pump. After the surgery, patients were transferred to the post-anesthetic care unit (PACU).
Outcome measurements
Before surgery, all patients were assessed for risk factors of PONV using Afpel scores [
5]. The primary outcome evaluated was analgesic efficacy, measured using the visual analogue scale (VAS). Other outcomes evaluated included the incidence rate of PONV, patient satisfaction with postoperative pain control, percentage of patients taking additional opioids, and the incidence rate of postoperative adverse effects. Nurses who were blinded to the group assignments were assigned to assess these outcomes. Patient satisfaction with postoperative pain control was assessed using a 5-point scale [
6], with 5 = very satisfied, 4 = somewhat satisfied, 3 = mixed (approximately equal satisfaction and dissatisfaction), 2 = somewhat dissatisfied, and 1 = very dissatisfied. If a patient requested additional pain control, 25–50 mg of pethidine was administered intramuscularly. Additional pethidine requirements within 48 h of surgery were documented and registered as the percentage of patients requiring additional opioids by the blinded nurses. The assessments of analgesic efficacy and incidence rate of PONV were performed preoperatively, on arrival in the PACU, and at 12, 24, and 48 h postoperatively.
Sample size
Postoperative pain at 24 h after the operation, as assessed by the VAS, was the primary outcome variable on which the sample size estimation was based. A previous study found that on postoperative day 1, the VAS was 1.55 ± 2.15 (mean ± standard deviation [SD]) after continuous intravenous injection of nefopam in post-abdominal surgery by laparotomy [
7]. We assumed a clinically significant minimum increase in the VAS at 24 h after the operation to be 1.5 (ɛ = 1.5). The sample size calculation for this study produced 26 patients per group when we considered type I (α) error = 0.05, type II (β) error = 0.20, SD (σ) = 2.15, and we added a predicted dropout rate of 10% and a patient per group to increase the power of test. There were 30 patients per group. The sample size was calculated according to the following equation.
σ (standard deviation) = 2.15,
ɛ (clinically significant minimum increase in the VAS at 24 h after the operation) = 1.5
µA - µB (difference in the VAS between the means of the two groups) = 0
Statistical analysis
Statistical analysis was performed using PASW Statistics version 18.0 for Windows (SPSS, Chicago, IL, USA). With the exception of patient numbers, ASA physical status, type of operation, percentage of patients requiring additional opioids, and the incidence rate of adverse effects, all measured values were reported as mean ± SD. Data distribution was evaluated using the Kolmogorov-Smirnov test. For continuous variables such as the VAS, the student t-test was employed to compare the intergroup difference. Chi-square test or Fisher's exact test were used for analyzing the risk factors of PONV, incidence of adverse effects, percentage of patients requiring additional opioids, and patient satisfaction with postoperative pain control. A difference was regarded as statistically significant at P < 0.05.
Results
Sixty of the enrolled patients completed the study (
Fig. 1). With respect to demographic data, there was no difference in age, ASA physical status, height, weight, and duration of operation between the two groups. There was no significant difference in the percentage of patients taking additional opioids between the two groups. The types of laparoscopic gynecologic surgeries performed in this study were diagnostic laparoscopy, myomectomy, ovarian cystectomy, salpingo-oophorectomy, and total hysterectomy. There were no statistically significant differences in the types of surgery between the groups (
Table 1).
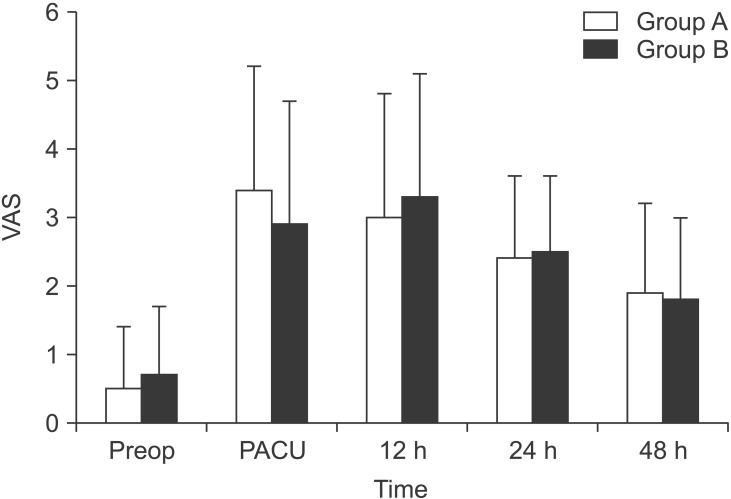
The postoperative VAS in the PACU, and at 12, 24, and 48 h after surgery was 3.4 ± 1.8, 3.0 ± 1.8, 2.4 ± 1.2, and 1.9 ± 1.3, respectively for group A, and 2.9 ± 1.8, 3.3 ± 1.8, 2.5 ± 1.1, and 1.8 ± 1.2, respectively for group B. The mean differences (95% confidence interval [CI]) of the postoperative VAS in the PACU, and at 12, 24, and 48 h after surgery were 0.50 (-0.43 to 1.43), -0.30 (-1.25 to 0.65), -0.05 (-0.65 to 0.55), and 0.10 (-0.55 to 0.75), respectively. All 95% CIs exceeded the non-inferiority margin of -1.5. Group B was not inferior to group A with respect to the postoperative VAS (
Fig. 2). Data PONV risk factors are shown in
Table 2. Risk factors such as gender, history of smoking, and history of PONV were not significantly different between the two groups. The incidence rate of postoperative nausea was lower in group B than in group A at 12 and 24 h after surgery (P = 0.004 and 0.017, respectively). In contrast, the incidence rate of vomiting was not statistically different between the two groups (
Table 3).
There was no significant difference in the incidence rate of adverse effects between the two groups (
Table 4). Patient satisfaction with postoperative pain control was also not significantly different between the two groups (
Table 5).
Discussion
IV-PCA, for postoperative pain control, may increase the overall patient satisfaction by minimizing respiratory failure, reducing respiratory system complications, and enhancing the analgesic effect [
8]. However, it may also increase the frequency and intensity of PONV when opioids are used [
9].
Nefopam is a non-opioid, non-steroidal centrally acting analgesic. Despite a lack of valid clinical trial data, it is used extensively in many European countries as an alternative to opioid analgesics for the relief of moderate to severe pain. The mechanism underlying its pharmacological action is not clear. It is suspected to induce analgesia using a mechanism similar to that of triple-receptor reuptake inhibitors, such as serotonin, norepinephrine, and dopamine [
10]. It also directly interacts with α2-adrenoceptors [
11] and is a non-competitive N-methyl-D-aspartate receptor antagonist [
12]. An injection of nefopam at the end of surgery has been shown to improve postoperative analgesia and reduce morphine requirement [
7]. However, other studies have been unable to confirm these results and the role of nefopam as an adjuvant to opioid analgesia in patients undergoing surgery has remained unconfirmed [
13].
Nefopam had been used as an adjunctive drug of IV-PCA with opioids in most studies. However, we used IV-PCA with nefopam alone because previous studies suggested that nefopam 20 mg was equipotent to morphine 6–12 mg and meperidine 50 mg [
34]. Therefore, we assumed that IV-PCA using nefopam alone would be sufficient to manage postoperative pain after laparoscopic gynecologic surgery and that this protocol may reduce the incidence of adverse effects such as dizziness, sedation, and PONV.
In the present study, IV-PCA using nefopam alone showed non-inferiority in analgesic efficacy, produced a lower incidence of PONV, and a higher patient satisfaction compared with IV-PCA using a combination of morphine and ketorolac after laparoscopic gynecologic surgery. These results suggest that it is possible to achieve effective analgesia and reduce PONV simultaneously in patients undergoing laparoscopic gynecologic surgery. Laparoscopic gynecologic surgery is generally less painful than open abdominal surgery. However, despite mild postoperative pain, many patients are still concerned about postoperative pain management.
Patients who undergo laparoscopic gynecologic surgery are at a high risk of PONV. The high risk factors of PONV are female gender, non-smoker, a history of PONV, and the use of opioids during surgery. If none, one, two, three, or four of these four risk factors are present, the incidence of PONV is 10%, 21%, 39%, 61%, and 79%, respectively [
5]. Our patients had relatively high Afpel scores and there was a high risk of PONV in both groups. The Afpel score for the group in which opioids were administered for IV-PCA was higher than that of the group in which nefopam was used. The mechanism of action of nefopam as an analgesic is known to be the inhibition of serotonin re-uptake, which can induce nausea and vomiting. Although nefopam has previously been associated with a 15–30% incidence of nausea, dizziness, and sweating [
14], our study showed that the incidence of nausea was lower at 12 and 24 h after surgery in the group that was administered nefopam alone for PCA than in the group that was administered morphine with ketorolac. A study on patients undergoing elective hepatic resection showed that the incidence of nausea was lower in a group receiving nefopam with morphine for PCA than in a group receiving morphine alone [
15]. Another study showed that, in patients undergoing thyroidectomy, postoperative nausea was lower in a nefopam-administered group in comparison with a ketorolacadministered group [
16]. In the present study, it is assumed that ramosetron 0.3 mg, the dose used to reduce PONV, alleviated most of the postoperative nausea within 12–24 h in both groups. However, after this period, the incidence rate of nausea in the nefopam-administered group was relatively lower than in the opioid-administered group. Our results also suggest that nefopam- induced PONV can be managed with antiemetics, such as serotonin reuptake inhibitors.
Nefopam is generally considered safe and well-tolerated. The reported adverse effects are mostly minor and include drowsiness, nausea, light-headedness, and sweating; these are not significant when an appropriate dose is administered. More serious potential adverse effects are confusion and tachycardia [
14]. Unlike non-steroidal anti-inflammatory drugs, nefopam has no effect on platelet function [
17] and in contrast to opioids, this drug does not appear to increase the risk of respiratory depression [
18]. In the present study, the incidence rates of adverse effects in the nefopam-administered group were 23.3% and 6.7% for dizziness and sweating, respectively. These rates were not significantly different between the opioid- and NSAID-administered groups.
This study suggests that IV-PCA using nefopam alone has an analgesic efficacy equivalent to that of morphine with ketorolac after laparoscopic gynecologic surgery. However, the intensity of the postoperative pain depends on the type of surgery; therefore, the results of this study are limited to laparoscopic gynecologic surgery. Therefore, further research on the appropriate dose of nefopam, the various types of opioids and their doses, in addition to the use of nefopam in different types of surgeries is required.
In conclusion, IV-PCA using nefopam alone has a non-inferior analgesic efficacy and produces a lower incidence of PONV in comparison with IV-PCA using a combination of morphine and ketorolac after laparoscopic gynecologic surgery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download