Abstract
Background
To investigate and analyze MRI findings in relation to visual analogue scale (VAS), Oswestry Disability Index (ODI), psychological-factor, sleep-quality, and Short-Form Health Survey (SF-36) scores among patients with central lumbar spinal stenosis (LSS) for the purpose of elucidating a correlation.
Methods
From July 2013 to May 2014, 117 consecutive patients with central LSS were included in this study. All of the MRIs were evaluated by one of the authors, and the evaluated items were the dural sac cross-sectional area (DSCSA), the number of stenotic levels, and the presence and levels of spondylolisthesis. The ODI, VAS, 36-item SF-36, Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), and Pittsburgh Sleep Quality Index (PSQI) questionnaires were used to evaluate the participants.
Results
There are no correlations between the ODI, VAS, BDI, BAI, PSQI, and SF-36 scores and the minimum DSCSA; however, a significant correlation was found between the ODI scores and multilevel LSS. The BDI, BAI, and PSQI scores are higher for multilevel LSS compared with single-level LSS, but the difference of this mean value is not statistically significant.
Go to : 
Lumbar spinal stenosis (LSS) is a commonly encountered, painful, and possibly disabling condition that is usually treated with a spinal epidural injection, or surgery for older patients [12]. Degenerative changes that include hypertrophy of the facet joint or surrounding ligamentous structure result in a narrowing of the spinal canal or the lateral nerve-root canals. Based on the most narrowed anatomical area, LSS is classified as either central, foraminal, or lateral [3].
The MRI technique is pivotal in the diagnosis of LSS, as it is reportedly more sensitive and specific in the identification of patients with LSS compared with computed tomography and myelography [234]. A direct measurement of the cross-sectional area of the dural sac is thought to be more effective for diagnosing central LSS than a measurement of the osseous spinal canal [5]; Sigmundsson et al. [5] used a cross-sectional area of 70 mm2 as the critical size for the diagnosis of LSS. Clinically, MRI findings provide a relatively objective way to visualize the narrowed spinal canal and are an important tool for preoperative or preinjection planning; however, in our clinical practice, we can encounter some patients whose anatomy shows a severely narrowed spinal canal with minimal or no corresponding symptoms. This phenomenon led us to question the relationship between a severe case of LSS that has been assessed in accordance with a radiographic MRI evaluation and a patient's symptoms.
According to previous reports, a significant correlation was not found between MRI findings and a patient's pain or disability levels [2567]; however, those studies usually used retrospective patient data that were obtained without a validated methodology to assess the patients' pain and disability, and psychosocial factors were not considered.
Well-validated questionaires including the Oswestry Disability Index (ODI) [8], Beck Depression Inventory (BDI) [9], Beck Anxiety Inventory (BAI) [10], Pittsburgh Sleep Quality Index (PSQI) [11], and the 36-item Short-Form Health Survey (SF-36) [12] have remarkably advanced the capability of the medical field to represent a patient's symptoms, level of disability, and psychosocial spectrum in an accurate manner.
In this study, we sought to investigate in a prospective setting the correlation between radiographic assessments of LSS severity using MRI, and assessments of LSS symptoms and psychosocial functions that were measured using standardized and well-validated questionnaires.
Go to : 
This study was approved by the ethics committee of our institution, and written, informed consent was obtained from all patients after we explained the risks, benefits, and study goals to them.
From July 2013 to May 2014, 117 consecutive patients with central LSS of at least a three-month duration were included. All patients had clinical symptoms with neurogenic claudication, persistent leg and/or back pain, a numbness or tingling sensation in one or both legs, and radiographic evidence of the compression of the cauda equina by degenerative changes of the ligamentum flavum, osteophytes, and facet joints. All of the included patients were scheduled to receive an epidural steroid injection due to their minimal responses to other conservative therapies. The following criteria resulted in exclusion: (1) previous spine surgery, (2) absence of lumbar MRI, (3) lumbar radiculopathy caused by degenerative disc diseases including herniated intervertebral disc disease (4) LSS of the foraminal or lateral types, (5) laboratory findings suggesting coagulopathy, infection, or inflammatory disease, and (6) patients who did not complete the self-reported questionnaire.
Thirty-five patients were excluded on the basis of the exclusion criteria, and 9 patients were excluded due to incomplete questionnaires, thereby resulting in a final total of 73 enrolled patients.
ODI, VAS, BDI, BAI, PSQI, and SF-36 scores were recorded for all of the patients.
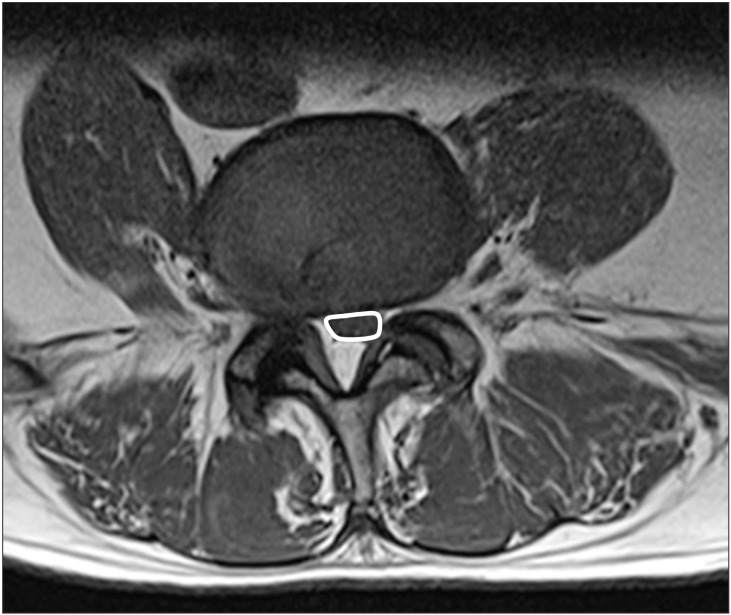
All of the MRIs were evaluated by one of the authors and one radiologist who were blinded to the clinical outcome and radiological report. The evaluated items included the dural sac cross-sectional area (DSCSA), the number of stenotic levels, and the presence and level of spondylolisthesis. We used the critical size of 100 mm2 as the objective diagnostic criteria for LSS. To calculate the DSCSA, we used a region of interest (ROI) curve on a diagnostic work station (Maro view version 5.4.10.57, Korea) (Fig. 1).
The DSCSA (mm2) was measured at the central part of the disc level on axial T1 images. We confirmed the classification of multilevel LSS if severe narrowing affected a patient on at least two levels in a visual analysis of the MRI. The minimum DSCSA was measured at the most stenotic level in cases of multilevel LSS and at one stenotic level in cases of single-level LSS. The level of spondylolisthesis were also identified, while 22 random cases, which formed a subset, were measured DSCSA independently by one of the authors and one radiologist, who also calculated the interclass correlation coefficient (ICC) together. Difficult measurements were discussed among the authors.
The ODI is a self-completed patient questionnaire that measures the levels of disability regarding the daily activities of 10 items to obtain a functional-level score. The ODI is advantageous for patients, as it is a self-assessment questionnaire that is easily comprehensible, and a wide domain of functions, pain, and daily living limitations have been included [2813]. We used the Korean-language version of the ODI which was validated by Jeon et al. [8]. The Korean-language version showed satisfactory reliability, and the Cronbach's alpha and test-retest correlation coefficients were 0.9168 and 0.9332, respectively [8].
The VAS scores for leg and back pain were obtained by measuring the distance (cm) from the origin of a horizontal line (total 10 cm) to the point indicated by the patient as an expression of their level of pain during the previous week; zero represented "no pain at all" and 10 represented "the worst pain imaginable."
The BDI is a 21-item, self-reported questionnaire that was designed to evaluate depression symptoms. The Korean-language BDI was used to evaluate depression between the different MRI findings, and was validated to show a significant, positive internal consistency (r = 0.88) and test-retest reliability (r = 0.60). A score of 16 or higher was suggested as the optimal cutoff value for the diagnosis of major depression in Korea [9].
The BAI is a 21-item, self-reported questionnaire that was designed to evaluate anxiety symptoms. Each question is on a four-point scale that adds up to a total score in the range from 0 to 63, with 0 representing no anxiety and 63 representing severe anxiety. A Korean-language version showed reliability and validity for both patients and the general population [10].
The PSQI is a 19-item, self-reported questionnaire that has been widely used to assess and monitor sleep patterns. The Korean-language version showed high reliability, and the Cronbach's alpha and test-retest correlation coefficients were 0.84 and 0.65, respectively. The contents of the PSQI include subjective sleep duration, sleep quality, sleep latency, habitual sleep efficiency, sleep distur-bances, use of sleep medication, and daytime dysfunction [11].
The Korean-language version of the SF-36 was used and it was validated as a reliable and credible questionnaire to measure the quality of life in the study groups [12]. The 36 items and scales were constructed using the Likert method of summated ratings. The 36 questions of the SF-36 covered the following eight categories: 1) physical functioning (walking, lifting); 2) role function-physical (limitation of ability to perform usual activities); 3) bodily pain (level of bodily pain or discomfort); 4) general health perceptions (global evaluation of health); 5) vitality (energy level or fatigue); 6) social functioning (impact of health or emotional problems on social activities); 7) role function-emotional (impact of emotional problems on work or daily activities); and 8) mental health (anxiety, depression, sense of psychological well-being). The first four categories were grouped as the physical component of the summary, while the last four categories were grouped as the mental component of the summary. The item scores for each category were coded, summed, and converted in accordance with a 0 to 100 scale, with 100 indicating the most favorable score.
We considered a two-tailed significance level of P = 0.05, whereby a power of 80% was used to detect a correlation coefficient of 0.27 [5], to determine that 65 patients were required.
All statistical evaluations were performed using both the Spearman and Pearson correlation analyses and an independent t-test with SPSS version 17.0 (SPSS, Chicago, IL, USA). The Spearman and Pearson correlation analyses were used to identify a correlation between the VAS, ODI, BDI, BAI, PSQI, and SF-36 scores and the radiographic MRI findings. The independent t-test was used to compare the mean values of the ODI, BDI, BAI, PSQI, and SF-36 questionnaires to those of the MRI findings.
Go to : 
The 73 participants in this study who had been diagnosed with a central LSS ranged in age from 26 years to 80 years, with a mean age of 57.0 years, and the sex composition was 32 men and 41 women.
A high level of correlation was found by the observers measuring the subset of 22 patients, with an ICC value of 0.925 (95% CI: 0.819-0.969, P < 0.001).
The mean of the minimum DSCSA was 65.8 mm2 (SD 14, range 16-115), with 66.7 mm2 (SD 13) for the women, and 64.7 mm2 (SD 15) for the men. The number of patients with a minimum DSCSA below 70 mm2 was 49, and it occurred most frequently at the L4-5 level (36 patients), followed by the L5-S1 level (19 patients). Twenty-four patients showed a DSCSA from 70 to 100 mm2. Among the 49 patients who showed a DSCSA < 70 mm2, 19 patients had two or more levels of LSS and 28 patients had one level of LSS. Nine patients had concomitant spondylolisthesis and 2 patients had spondylolisthesis at more than one level. Spondylolisthesis was most frequently found at the L4-5 level, and was more common in the women (7) than the men (2). The mean age of the patients with spondylolisthesis was 63.3 years (SD 3.4) (Table 1).
We found no correlation between the ODI, BDI, BAI, PSQI, and SF-36 values and the minimum DSCSA; however, we found a significant correlation between the ODI values and multilevel LSS (r = 0.236, P = 0.045, Table 2).
The mean ODI scores for the multilevel and single-level stenoses are 20.1 and 23.8, respectively, and the difference of this mean value is statistically significant (P = 0.040). The BDI, BAI, and PSQI scores are higher in the multilevel LSS cases compared with the single-level LSS cases, but the difference of this mean value is not statistically significant (Table 3).
Significant differences were not found between those patients with spondylolisthesis and those without spondylolisthesis regarding the mean values of the minimum DSCSA and the ODI, BDI, BAI, PSQI, and SF-36 questionnaires (Table 4).
Go to : 
The main finding of our study is that a linear correlation is not evident between radiographically assessed LSS and clinical symptoms that included psychosocial factors. We did, however, find a significant correlation between the ODI scores and multilevel LSS, whereas no such correlation was found for DSCSA. With an increase of the number of stenosis levels, an increasingly severe disability was observed in the patients with LSS; however, previous studies on the correlation between ODI and the severity of LSS showed conflicting results. The LSS severity that was identified through the measurement of the sagittal diameter of the dural sac significantly predicted disability, even after adjusting for age, sex, therapy regimen, and body mass [14]; however, various authors reported a poor correlation between radiographically assessed LSS and clinical findings, severity of symptoms, or ODI scores [21516].
We assessed the degree of narrowing by measuring the DSCSA of the MRIs; however, the dynamic nature of the narrowing extent is prone to change according to the posture of the patient. Hirasawa et al. [17] reported a posture-dependent difference of the DSCSA in asymptomatic volunteers and, depending on the posture, the mean DSCSA and anteroposterior dural sac diameter changed dynamically. The capability to predict the severity of the patient's symptoms using a static MRI image of the canal dimension was therefore somewhat limited.
Our results indicate that LSS is not simply an anatomical disorder that can be explained by MRI, but that this disease may have underlying, dynamic, and more complex mechanisms to be explored. The spontaneous, adaptive resolution of pain and disability over time could possibly explain the poor correlation between radiographically assessed LSS and clinical findings.
Yukawa et al. [18] reported that patients with multilevel central LSS walked on the treadmill for a significantly shorter distance both preoperatively and postoperatively compared with those patients with LSS at only one level. They also found significant correlations among the patient age, the number of stenotic levels, and walking capacity during the treadmill test.
Sigmundsson et al. [5], however, reported that patients with multilevel LSS scored significantly better for the general health items of the SF-36, and experienced less leg and back pain despite having smaller DSCSAs than patients with single-level LSS. Similarly, Kuittinen et al. [19] also reported that patients with severe LSS at only one level achieved shorter walking distances than patients with severe multilevel LSS.
Our results also showed that the multilevel LSS patients had higher BDI, BAI, and PSQI scores compared with those patients with single-level LSS, although there are no statistical differences. Further, Pakarinen et al. [20] demonstrated that depressive patients were associated with poorer surgical outcomes when they were evaluated using ODI scores.
In our previous study, we demonstrated that patients with LSS who experienced chronic lower-back pain showed considerable functional disability with psychological impairment; moreover, a significant correlation was shown between the BDI, BAI, PSQI, and SF-36 scores and the ODI score [13]. In this prospective study, we also assessed the psychological status (BDI, BAI), sleep quality (PSQI), and health-associated life quality (SF-36) of the patients with well-validated questionnaires, but we did not find any straightforward relationships between these psychological and general health data and the radiographic findings.
Epidural steroid injections have been used for a number of decades in the treatment of radicular pain caused by nerve-root compression or LSS after the failure of conservative treatments including oral medications and physical therapy [1212223]. In this study, every enrolled patient did not have radiculopathy due to central LSS, but they had predominant leg pain or tingling sensations from intermittent neurogenic claudication. We therefore used an epidural steroid injection to treat the claudication, and significant pain relief was reported by those patients with central LSS who received the caudal epidural steroid injections [24].
Degenerative spondylolisthesis is highly related to degenerative facet-joint arthritis and occurs most frequently at the L4-5 level with a female predominance. The superior vertebra slides forward onto the inferior one, causing a constricted central canal and lateral recess that ultimately lead to a variety of clinical symptoms [22]. Our results show that degenerative spondylolisthesis was most frequently found at L4-5, and 7 of the 9 patients with degenerative spondylolisthesis were female; additionally, those patients with degenerative spondylolisthesis did not have more narrowed spinal canals. The minimum DSCSA was 71.6 mm2 at the sliding level in those patients with spondylolisthesis, compared with 65.0 mm2 at the most stenotic level in patients without spondylolisthesis. Sirvanci et al. [2] reported that a more pronounced narrowing of the spinal canal was not observed in patients with degenerative spondylolisthesis.
A strength of this study is that it was conducted with wellvalidated questionnaires that were designed to represent a patient's physical and psychological well-being.
This study has a patient-selection bias, as we enrolled only those patients who had planned for an epidural steroid injection, and most of the patients (67.1%) had a DSCSA less than 70 mm2. We might have found a significant correlation between MRI and level of disability or pain if we included healthy volunteers and patients with a mild LSS.
A limitation in our study is a small sample size that may limit our statistical power to detect a small correlation coefficient, although we consider that our sample would be sufficient to identify correlation between MRI findings and clinical symptoms. We recognize that future studies increasing the sample size should be performed, with our results being useful as a referent for MRI findings probably do not correlated with clinical findings. Further studies in other populations are required to confirm our findings.
Another limitation of this study is the routine method of performing a clinical MRI that is currently used, whereby patients must lie down in the supine position, as a patient's symptoms may worsen in a standing position, which could also alter the anatomy of the neural canal. Accordingly, a standing position would be the most proper image-acquisition method for associating image findings with a patient's symptoms.
In conclusion, we could not find any linear or straightforward correlations between radiographically assessed LSS and a group of clinical symptoms that included psychosocial factors; however, a significant correlation was found between ODI scores and multilevel LSS.
Go to : 
References
1. Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Sullivan SD, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014; 371:11–21. PMID: 24988555.

2. Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008; 17:679–685. PMID: 18324426.

3. Kobayashi S. Pathophysiology, diagnosis and treatment of intermittent claudication in patients with lumbar canal stenosis. World J Orthop. 2014; 5:134–145. PMID: 24829876.

4. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014; 119:541–549. PMID: 24365318.

5. Sigmundsson FG, Kang XP, Jonsson B, Stromqvist B. Correlation between disability and MRI findings in lumbar spinal stenosis: a prospective study of 109 patients operated on by decompression. Acta Orthop. 2011; 82:204–210. PMID: 21434811.
6. Geisser ME, Haig AJ, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, et al. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin J Pain. 2007; 23:780–785. PMID: 18075405.

7. Haig AJ, Geisser ME, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, et al. Electromyographic and magnetic resonance imaging to predict lumbar stenosis, low-back pain, and no back symptoms. J Bone Joint Surg Am. 2007; 89:358–366. PMID: 17272451.

8. Jeon CH, Kim DJ, Kim SK, Kim DJ, Lee HM, Park HJ. Validation in the cross-cultural adaptation of the Korean version of the Oswestry Disability Index. J Korean Med Sci. 2006; 21:1092–1097. PMID: 17179693.

9. Jo SA, Park MH, Jo I, Ryu SH, Han C. Usefulness of Beck Depression Inventory (BDI) in the Korean elderly population. Int J Geriatr Psychiatry. 2007; 22:218–223. PMID: 17044132.

10. Lee EH, Kim JH, Yu BH. Reliability and validity of the self-report version of the Panic Disorder Severity Scale in Korea. Depress Anxiety. 2009; 26:E120–E123. PMID: 19373866.

11. Sohn SI, Kim do H, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012; 16:803–812. PMID: 21901299.

12. Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M. Development of the Korean version of Short-Form 36-Item Health Survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med. 2004; 203:189–194. PMID: 15240928.

13. Hong JH, Kim HD, Shin HH, Huh B. Assessment of depression, anxiety, sleep disturbance, and quality of life in patients with chronic low back pain in Korea. Korean J Anesthesiol. 2014; 66:444–450. PMID: 25006368.

14. Hurri H, Slätis P, Soini J, Tallroth K, Alaranta H, Laine T, et al. Lumbar spinal stenosis: assessment of long-term outcome 12 years after operative and conservative treatment. J Spinal Disord. 1998; 11:110–115. PMID: 9588466.
15. Lohman CM, Tallroth K, Kettunen JA, Lindgren KA. Comparison of radiologic signs and clinical symptoms of spinal stenosis. Spine (Phila Pa 1976). 2006; 31:1834–1840. PMID: 16845360.

16. Jönsson B, Annertz M, Sjöberg C, Strömqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part I: Clinical features related to radiographic findings. Spine (Phila Pa 1976). 1997; 22:2932–2937. PMID: 9431629.
17. Hirasawa Y, Bashir WA, Smith FW, Magnusson ML, Pope MH, Takahashi K. Postural changes of the dural sac in the lumbar spines of asymptomatic individuals using positional stand-up magnetic resonance imaging. Spine (Phila Pa 1976). 2007; 32:E136–E140. PMID: 17304123.

18. Yukawa Y, Lenke LG, Tenhula J, Bridwell KH, Riew KD, Blanke K. A comprehensive study of patients with surgically treated lumbar spinal stenosis with neurogenic claudication. J Bone Joint Surg Am. 2002; 84-A:1954–1959. PMID: 12429755.

19. Kuittinen P, Sipola P, Saari T, Aalto TJ, Sinikallio S, Savolainen S, et al. Visually assessed severity of lumbar spinal canal stenosis is paradoxically associated with leg pain and objective walking ability. BMC Musculoskeletal Disord. 2014; 15:348.

20. Pakarinen M, Vanhanen S, Sinikallio S, Aalto T, Lehto SM, Airaksinen O, et al. Depressive burden is associated with a poorer surgical outcome among lumbar spinal stenosis patients: a 5-year follow-up study. Spine J. 2014; 14:2392–2396. PMID: 24486473.

21. Benny BV, Patel MY. Predicting epidural steroid injections with laboratory markers and imaging techniques. Spine J. 2014; 14:2500–2508. PMID: 24743064.

22. Kraiwattanapong C, Wechmongkolgorn S, Chatriyanuyok B, Woratanarat P, Udomsubpayakul U, Chanplakorn P, et al. Outcomes of fluoroscopically guided lumbar transforaminal epidural steroid injections in degenerative lumbar spondylolisthesis patients. Asian Spine J. 2014; 8:119–128. PMID: 24761192.

23. Ploumis A, Christodoulou P, Wood KB, Varvarousis D, Sarni JL, Beris A. Caudal vs transforaminal epidural steroid injections as short-term (6 months) pain relief in lumbar spinal stenosis patients with sciatica. Pain Med. 2014; 15:379–385. PMID: 24341966.

24. Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician. 2012; 15:371–384. PMID: 22996849.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download