Abstract
Background
Microvascular decompression with retromastoid craniotomy carries an especially high risk of postoperative nausea and vomiting. In this study, we compare the antiemetic efficacy of ramosetron and ondansetron in patients undergoing microvascular decompression with retromastoid craniotomy.
Methods
Using balanced anesthesia with sevoflurane and remifentanil infusion, ondansetron 8 mg (group O, n = 31) or ramosetron 0.3 mg (group R, n = 31) was administered at the dural closure. The incidence and severity of postoperative nausea and vomiting, required rescue medications and the incidence of side effects were measured at post-anesthetic care unit, 6, 24 and 48 hours postoperatively. Independent t-tests and the chi-square test or Fisher's exact test were used for statistical analyses.
Results
There were no differences in the demographic data between groups, except for a slightly longer anesthetic duration of group R (P = 0.01). The overall postoperative 48 hour incidences of nausea and vomiting were 93.6 and 61.3% (group O), and 87.1 and 51.6% (group R), respectively. Patients in group R showed a less severe degree of nausea (P = 0.02) and a lower incidence of dizziness (P = 0.04) between 6 and 24 hours.
Conclusions
The preventive efficacy of ramosetron when used for postoperative nausea and vomiting was similar to that of ondansetron up to 48 hours after surgery in patients undergoing microvascular decompression with retromastoid craniotomy. A larger randomized controlled trial is needed to confirm our findings.
Postoperative nausea and vomiting (PONV) is one of the most common postoperative discomforts, most likely resulting in serious complications consequent to elevated intracranial and arterial pressure and leading to a catastrophic postoperative course, especially in neurosurgical patients [1]. Various pathways through the vomiting center, including vagal afferents of the gastrointestinal tract, the chemoreceptor trigger zone in the area postrema, and the vestibular system, can induce PONV [2]. An effective prophylactic antiemetic regimen is mandatory for patients undergoing craniotomy, which is regarded as a highrisk procedure for PONV. Along with infratentorial craniotomy, suggested as an additional independent risk factor of PONV [34], recent retrospective studies have reported that microvascular decompression (MVD) is an especially strong risk factor for PONV among craniotomy patients, with reported odds ratios ranging from 5.38 [5] to 6.7 [6]. The incidence of PONV after MVD with retromastoid craniotomy (RMC) is higher than 60% within 24 hours postoperatively despite the use of ondansetron [3]. The higher incidence of PONV during MVD is attributed to medial retraction of the cerebellum performed near the area postrema or the chemoreceptor trigger zone [7].
Ondansetron, a 5-hydroxytryptamine3 (5-HT3) receptor antagonist, is widely used and known to be effective for PONV [8]. Ramosetron, a selective serotonin 5-HT3 receptor antagonist with a higher affinity, is also effective for preventing PONV [9]. A meta-analysis of the antiemetic efficacy of ondansetron after craniotomy [10] revealed that ondansetron significantly reduces nausea and vomiting in adult patients by 22 and 57% within 24 hours after surgery, respectively, whereas it is not effective between 24 and 48 hours after surgery. On the other hand, a randomized prospective double-blind study [11] found that nausea after infratentorial craniotomy exhibited a bimodal pattern up to 48 hours, suggesting that a prophylactic antiemetic treatment for PONV is needed up to 48 hours postoperatively. In a clinical trial, the efficacy of ramosetron was well maintained during a 48 hour period such that it was significantly higher with ramosetron for nausea and vomiting 6-48 hours after treatment [9]. Therefore, ramosetron may be more appropriate than ondansetron with regard to the complete response and incidence of PONV until 48 hours after surgery in some patients at high risk for PONV [121314].
The aim of this study was to compare the antiemetic efficacy of ramosetron with that of ondansetron up to 48 hours after surgery in patients undergoing MVD with RMC.
After approval by the Institutional Review Board (4-2010-0242) and written informed consent, 64 adult patients aged 20-75 years and with an American Society of Anesthesiologists physical status I or II who were scheduled for MVD with RMC were included in this prospective study. Exclusion criteria included patients with pregnancy; those having undergone chemotherapy or ventriculo-peritoneal shunt insertion; those with an allergy to ondansetron or ramosetron; those having undergone antiemetic therapy within 24 hours before the operation; those having systemic steroid therapy within 24 hours before the operation or up to 48 hours during the postoperative period, those having had an emergency operation; and those with cardiovascular disease, respiratory disease, renal disease, or hepatic disease. Additionally, those with a Glasgow Coma Scale Score of less than 13 points were excluded.
Patients were randomly allocated to receive ondansetron (group O) or ramosetron (group R) according to a computer grouping program. None of the patients received any premedication, and all were asked to provide a detailed medical history and a current list of medications.
Anesthesia and monitoring were standardized for all patients. Pulse oximetry, electrocardiogram, non-invasive blood pressure and end-tidal CO2 were continuously monitored in the operating room. General anesthesia was induced with a bolus of propofol 2.0 mg/kg and remifentanil 0.5-1 µg/kg. Rocuronium 0.8 mg/kg was administered for endotracheal intubation. Anesthesia was maintained with sevoflurane (age-adjusted minimum alveolar concentration of 0.6-0.9 in air along with 50% oxygen), supplemented with remifentanil infusion at 0.05-0.2 µg/kg/min. Controlled ventilation was performed to maintain an end-tidal CO2 level of 32-35 mmHg during surgery.
At the onset of dural closure, 8 mg of ondansetron (Onseran®, Yuhan Corp., Seoul, Korea) or 0.3 mg of ramosetron (Nasea®, Astellas, Tokyo, Japan) was administered intravenously. The study medication was prepared by one of the investigators (Ha, SH) in identical 5 ml syringes and administered at an equal volume of 4 ml (ramosetron was prepared with 2 ml of normal saline). The other investigators were unaware of which drug was being administered to the patients. Fentanyl 50 µg was administered during skin closure for postoperative analgesia. At the end of the surgery, sevoflurane and remifentanil infusion were discontinued. After adequate reversal of the neuromuscular blockade with glycopyrrolate 0.2 mg and pyridostigmine 0.2 mg/kg, all patients were extubated and observed in the post-anesthetic care unit (PACU) for approximately one hour before being transferred to the general ward of the hospital.
Investigators who were unaware of the patient treatment group evaluated the occurrence and severity of nausea, the occurrence of vomiting, pain intensity levels, and the requirements of rescue antiemetics or analgesics at the PACU at intervals of 1-6 hours, 6-24 hours and 24-48 hours after surgery. The occurrence of side effects of the 5-HT3 antagonist, such as dizziness and sedation, were also assessed. Nausea was defined as a subjectively unpleasant sensation associated with the awareness of an urge to vomit, while vomiting was defined as a single episode of the forceful expulsion of gastric contents through the mouth. Retching, defined as the expulsive movement of the stomach muscles without the expulsion of stomach contents, was also considered as vomiting. The intensity of nausea was graded using a verbal 11-point rating scale, with 0 indicating no nausea and 10 indicating the worst nausea. The severity of nausea was graded according to verbal rating scale scores: no (0), mild (1-3), moderate (4-6) and severe (7-10). The patients were asked whether they felt any occurrence of dizziness and/or sedation during the period of study. Metoclopramide 10 mg was given intravenously as a rescue antiemetic when the patient asked to be treated for nausea or if vomiting more than twice within a 15 minute period. Pain intensity scores were measured on a visual analog scale in cm, ranging from 0 (no pain) to 10 (the worst pain imaginable). Patients received tramadol at 50 mg intravenously if they complained of pain greater than 5 on the visual analog scale. All adverse events were reviewed, judged and recorded by the investigator.
This prospective investigation was performed as a preliminary study in nature because there were no previous reports about antiemetic efficacy either with ondansetron or ramosetron in patients undergoing MVD with RMC. Thus, the authors intended to include at least 30 patients in each group to pass the normality test. For statistical analysis, independent two-sample t-tests and chi-square or Fisher's exact tests were performed to compare continuous and categorical data, respectively. A P value of less than 0.05 was considered statistically significant. All analyses were performed using SAS (version 9.2, SAS Inc., Cary, NC, USA).
Sixty-two patients (31 patients in each group) among the 64 patients enrolled in the study were analyzed because two patients (1 in group O and 1 in group R) violated the experimental protocol. Demographic data showed no statistical significance between the two groups, but the anesthetic duration in group R was longer than that in group O (231 ± 41 minutes vs. 261 ± 53 minutes, P = 0.01, Table 1).
The overall incidence rates of nausea and vomiting 48 hours postoperative were 93.6 and 61.3% in group O and 87.1 and 51.6% patients in group R, respectively and there were no statistical differences. Nearly 80% of the patients regardless of their group required a rescue antiemetic. None of the measured variables were different statistically between the two groups at any measured interval with regard to the incidence of nausea, vomiting, required antiemetics, pain scores and required rescue analgesics (Tables 2 and 3). However, between 6 and 24 hours postoperatively, more patients in group O tended to experience moderate to severe nausea (58.1 vs. 29.0%, P = 0.02; Fig. 1) and dizziness (54.8 vs. 29.0%, P = 0.04; Table 4) compared with those in group R. When matching the anesthesia time, a comparison of the two groups showed that the incidence of dizziness was similar to that in the unmatched data.
This prospective randomized observer-blind preliminary study was performed to compare the antiemetic efficacy of ramosetron with that of ondansetron in patients undergoing MVD with RMC, which is an an extremely high-risk for PONV [356] until 48 hours after surgery. Unfortunately, although ramosetron tended to reduce the severity of nausea and the incidence of dizziness between 6 and 24 hours as compared with ondansetron, the antiemetic efficacy of ramosetron was similar to that of ondansetron and insufficient up to 48 hours after surgery.
The ondansetron group in this study exhibited higher overall incidences of nausea (93.6%) and vomiting (61.3%) in comparison with a retrospective meta-analysis of craniotomy in adult patients [1015]. In fact, a retrospective analysis may underestimate the incidence of PONV unless patients complained of a mild degree of nausea. As a secondary aim of this study regarding how long antiemetic medications should be given prophylactically for these patients, many patients in both group O and group R remained nauseated (54.8 and 35.5%) and vomited (16.1 and 9.7%) between 24 and 48 hours, suggesting that an antiemetic regimen should be considered at least postoperative for 48 hours. Our results are comparable to those in a previous study involving infratentorial craniotomy [11], in which nearly 50% of patients experienced nausea and 20% of patients vomited during 48 hours postoperatively despite the use of ondansetron at 8 mg.
Through a blockade of receptors in the chemoreceptor trigger zone before emetic stimuli associated with anesthesia and surgery, ondansetron provides greater antiemetic efficacy and prevents emesis for more than 24 hours [8]. However, the antiemetic efficacy of the ondansetron is mostly limited to an antivomiting effect with less of an anti-nausea effect [16]. Being comparable to the controversy regarding the effects of ondansetron on PONV after craniotomy in adult patients [1015], ramosetron, a selective 5-HT3 receptor antagonist with higher affinity to that receptor and a longer duration of action than ondansetron, was not sufficient in preventing PONV in patients undergoing MVD with RMC, as 87.1 and 51.6% of patients here experienced nausea and vomiting up to 48 hours after surgery, respectively. These results suggest that ramosetron alone may be too weak to prevent PONV in extremely high-risk patients, although a better antiemetic effect of ramosetron compared to ondansetron was evident in less susceptible surgical circumstances up to 48 hours postoperatively [9].
Nevertheless, ramosetron reduced the severity of nausea and the frequency of dizziness between 6 and 24 hours after surgery (P = 0.02, P = 0.04, respectively). In addition, although not statistically significant, the incidence rates of nausea and vomiting with ramosetron were less than those for ondansetron between 24 and 48 hours after surgery.
Although ramosetron was superior to ondansetron in preventing PONV in patients undergoing other highly susceptible surgical circumstances, such as lumbar spine surgery [12] or unilateral total knee replacement [13], we did not observe a satisfactory antiemetic effect with ramosetron in patients undergoing MVD with RMC. Thus, we could consider possible reasons why MVD with RMC is associated with significantly increased and prolonged risk of PONV. As suggested by Eberhart et al. [17], blood clots or air around the surgical sites may trigger the nearby area postrema, located in the vomiting center. Specifically, the reduction rate of pneumocephalus after craniotomy, an unavoidable sequela of craniotomy, may provide insight as to why an antiemetic plan should be in place during the first 48 hours after surgery, as pneumocephalus resolves by 31% per day after craniotomy [18].
There are several possible limitations of this study. First, we did not compare the antiemetic efficacy of ramosetron to that of a placebo control. However, a study design with a placebo control group would be inappropriate because the subjects were at an extremely high risk for PONV. Secondly, the anesthetic duration in group R lasted slightly longer than that in group O (261 ± 53 vs. 231 ± 41 minutes, P = 0.01). However, we considered that the small difference of 30 minutes would not significantly affect the antiemetic efficacy of ramosetron. Of importance, lastly, the sample size of our preliminary study may have been too small to detect statistical significance in the differences between ramosetron and ondansetron. In fact, clinical trials for especially high-risk patients for PONV require as few patients as possible to be enrolled, as severe nausea or vomiting after craniotomy is associated with higher morbidity and mortality rates as well as higher medical expenses.
In conclusion, ramosetron and ondansetron showed no significant differences with regard to the preventive efficacy of PONV after MVD with RMC. However, considering the pilot nature of this study, the results here are not definitive. A multimodal antiemetic approach can be considered for at least 48 hours after MVD with RMC, as the efficacy of using ramosetron alone may not be enough to prevent PONV in patients undergoing MVD with RMC. In addition, the development of a therapeutic regimen for antiemesis will be necessary.
Acknowledgments
The authors thank to Hye Sun Lee, Biostatistician, Department of Research Affairs, Yonsei University College of Medicine, for statistical analysis.
References
1. Habib AS, Keifer JC, Borel CO, White WD, Gan TJ. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011; 112:813–818. PMID: 21081776.

2. Andrews PL. Physiology of nausea and vomiting. Br J Anaesth. 1992; 69(7 Suppl 1):2S–19S. PMID: 1486009.

3. Meng L, Quinlan JJ. Assessing risk factors for postoperative nausea and vomiting: a retrospective study in patients undergoing retromastoid craniectomy with microvascular decompression of cranial nerves. J Neurosurg Anesthesiol. 2006; 18:235–239. PMID: 17006120.

4. Latz B, Mordhorst C, Kerz T, Schmidt A, Schneider A, Wisser G, et al. Postoperative nausea and vomiting in patients after craniotomy: incidence and risk factors. J Neurosurg. 2011; 114:491–496. PMID: 21029035.

5. Sato K, Sai S, Adachi T. Is microvascular decompression surgery a high risk for postoperative nausea and vomiting in patients undergoing craniotomy? J Anesth. 2013; 27:725–730. PMID: 23649917.

6. Tan C, Ries CR, Mayson K, Gharapetian A, Griesdale DE. Indication for surgery and the risk of postoperative nausea and vomiting after craniotomy: a case-control study. J Neurosurg Anesthesiol. 2012; 24:325–330. PMID: 22828153.
7. Seubert CN, Mahla ME. Neurologic monitoring. In : Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone Elsevier;2010. p. 1477–1514.
8. Claybon L. Single dose intravenous ondansetron for the 24-hour treatment of postoperative nausea and vomiting. Anaesthesia. 1994; 49(Suppl):24–29. PMID: 8129159.

9. Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc). 2002; 38:75–89. PMID: 12532186.

10. Frost F, Dailler F, Duflo F. Ondansetron: a meta-analysis on its efficacy to prevent postoperative nausea and vomiting after craniotomy in adults and children. Ann Fr Anesth Reanim. 2010; 29:19–24. PMID: 20080017.
11. Fabling JM, Gan TJ, El-Moalem HE, Warner DS, Borel CO. A randomized, double-blind comparison of ondansetron versus placebo for prevention of nausea and vomiting after infratentorial craniotomy. J Neurosurg Anesthesiol. 2002; 14:102–107. PMID: 11907389.

12. Choi YS, Shim JK, Yoon DH, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976). 2008; 33:E602–E606. PMID: 18670328.
13. Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010; 65:500–504. PMID: 20337618.

14. Lee JW, Park HJ, Choi J, Park SJ, Kang H, Kim EG. Comparison of ramosetron's and ondansetron's preventive anti-emetic effects in highly susceptible patients undergoing abdominal hysterectomy. Korean J Anesthesiol. 2011; 61:488–492. PMID: 22220226.

15. Neufeld SM, Newburn-Cook CV. The efficacy of 5-HT3 receptor antagonists for the prevention of postoperative nausea and vomiting after craniotomy: a meta-analysis. J Neurosurg Anesthesiol. 2007; 19:10–17. PMID: 17198095.

16. Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997; 87:1277–1289. PMID: 9416710.
17. Eberhart LH, Morin AM, Kranke P, Missaghi NB, Durieux ME, Himmelseher S. Prevention and control of postoperative nausea and vomiting in post-craniotomy patients. Best Pract Res Clin Anaesthesiol. 2007; 21:575–593. PMID: 18286838.

18. Gore PA, Maan H, Chang S, Pitt AM, Spetzler RF, Nakaji P. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg. 2008; 108:926–929. PMID: 18447708.

Fig. 1
Severity of nausea at PACU, at intervals of 1-6 hours, 6-24 hours and 24-48 hours after surgery. The severity of nausea was graded according to verbal 11-point rating scale scores (0 = no, 10 = worst): no (0), mild (1-3), moderate (4-6) and severe (7-10). P value was analyzed using Fisher's exact test. O: ondansetron group, R: ramosetron group, h: hours, mod: moderate, PACU: post anesthetic care unit.
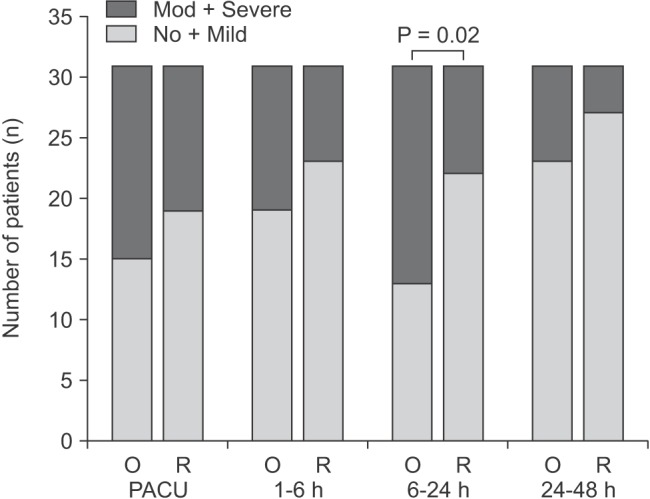
Table 1
Demographic Data
Data are mean ± SD or number of patients (%). P value was analyzed using either of independent two sample t-test or chi-square test. Group O: ondansetron group, Group R: ramosetron group, ASA PS: American Society of Anesthesiologists Physical Status, DM: diabetes mellitus, PONV: postoperative nausea and vomiting. *P < 0.05.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download