Abstract
Background
Sevoflurane exposure during the early postnatal period causes neuroinflammation and neuronal apoptosis in rodents. Bone marrow stromal cells (BMSCs) have been shown to protect and repair the damaged central nervous system, for example in ischemic stroke models. In this study, we investigated whether intravenous administration of BMSCs ameliorated neurodegeneration, induced by sevoflurane exposure, in neonatal rats.
Methods
Sprague-Dawley rat pups (postnatal day 7) were exposed to 2% sevoflurane for 6 h (vehicle group, n = 7). BMSCs were administered 30 min after induction of sevoflurane anesthesia (BMSCs group, n = 7). The pups were exposed to carrier gas only, as a negative control (mock anesthesia group, n = 4). We assessed the therapeutic effects of BMSC treatment by measuring expression of the pro-inflammatory cytokine interleukin-6 (IL-6), and levels of cleaved caspase-3, in brain tissues immediately following sevoflurane anesthesia.
Results
Analysis of the cleaved caspase-3 bands revealed that levels of activated caspase-3 were elevated in the vehicle group compared with the mock anesthesia group, indicating that a single exposure to sevoflurane at subclinical concentrations can precipitate neuronal apoptosis. BMSC treatment did not suppress apoptosis induced by sevoflurane exposure (compared with the vehicle group). The vehicle group had higher proinflammatory cytokine IL-6 protein levels compared with the mock anesthesia group, indicating that sevoflurane exposure induces IL-6 expression. BMSC treatment suppressed sevoflurane-induced increases in IL-6 expression, indicating that these cells can inhibit the neuroinflammation induced by sevoflurane exposure (vehicle group vs. BMSC group).
Prolonged exposure to anesthetics, in neonatal rodents and nonhuman primates, triggers widespread apoptotic neural death in the brain, and consequent long-term cognitive impairment. [12345] Although the mechanisms mediating the neurotoxicity of anesthetics in the neurodevelopmental stage are essentially unknown, recent studies have shown that neurodegeneration induced by anesthetics is a complex process involving organellar dysfunction [6], reduced expression of brain-derived neurotrophic factor [7], activation of death-inducing signaling pathways [8] and neuroinflammation [910]. Sevoflurane, commonly used in obstetric and pediatric anesthesia, has been reported to induce neuronal apoptosis, and long-term cognitive impairment, in neonatal rodents [11]. Although little is known about the neurotoxic effects of sevoflurane exposure, several studies have demonstrated that sevoflurane-induced neurotoxicity during the developmental stage can be prevented by certain therapeutic strategies [12131415].
Recent studies have highlighted the potential utility of bone marrow stromal cells (BMSCs) in protecting against central nervous system injury [161718]. BMSCs are capable of diverging into various mesenchymal lineages, including osteoblasts, chondroblasts, adipocytes, myocytes and fibroblasts, as well as non-mesenchymal lineages, including neuronal and glial cells. BMSCs also have the capacity to pass through the blood-brain barrier [19]. Intravenously administered BMSCs can migrate to the brain and secrete various cytokines and growth factors that have immunomodulatory, angiogenic, anti-inflammatory and anti-apoptotic effects, which can help alleviate brain damage [20]. These properties suggest that BMSCs may have therapeutic potential for sevoflurane neurotoxicity. In this study, we investigated whether intravenously administered BMSCs, following sevoflurane induction, had the capacity to alleviate sevofluraneinduced apoptosis in neonatal rats.
All experiments were approved by the Animal Care and Use Committee of Tokyo Medical and Dental University. Every effort was made to minimize the number of animals used.
BMSCs were isolated according to the Caplan method [21]. Briefly, following deep anesthesia and euthanization of adult male Sprague-Dawley rats (weight = 180 g), femurs and tibias were extracted bilaterally, and distal epiphyses were removed. A 22-G needle was inserted into the proximal end of the bone, and marrow was flushed into sterile cryotubes and stored on ice. The entire bone marrow suspension was cultured in 10-cm culture dishes. The cells were then cultured in Dulbecco's modified Eagle's medium, containing 10% fetal bovine serum and antibiotics (100 U/ml penicillin, 100 µg/ml streptomycin and 10 µg/ml gentamicin). After 3 days, tightly adhered cells were trypsinized, resuspended with fresh medium and transferred to new culture dishes. Cells were grown to confluence and used at passages 3-10. For intravenous administration, BMSCs were harvested and resuspended in phosphate-buffered saline (PBS) at 1 × 107 cells/ml. The cells were placed on ice prior to intravenous administration. Fifty microliters of BMSCs were injected via the jugular vein, using a 29-G 1/2" sterilized insulin syringe, within 30 min of induction of sevoflurane anesthesia. An identical volume of PBS was injected in the vehicle group.
We followed a slightly modified version of Satomoto et al. [11]'s protocol for sevoflurane anesthesia. Briefly, postnatal day 7 Sprague-Dawley rat pups (body weight approximately 14 g) were placed in a humid chamber (250 × 250 × 300 mm) with a warm mat heated to 38 ± 1℃. Two percentage sevoflurane was delivered using a calibrated flowmeter (Shinano, Tokyo, Japan). The total gas flow was 1 L/min, with 40% O2 used as the carrier gas. The rat pups were divided into three groups: pups exposed to sevoflurane for 6 h, with vehicle administration (vehicle group, n = 7); pups exposed to sevoflurane for 6 h, with BMSCs administration (BMSCs group, n = 7); and pups exposed to carrier gas only (i.e., without sevoflurane) for 6 h (mock anesthesia group, n = 4). This latter group represented a negative control.
Immediately following the 6 h period of sevoflurane or mock anesthesia, brains were removed and homogenized in 100 µl homogenization buffer containing 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM Na4P2O7, and a protease inhibitor cocktail. Following centrifugation at 14,000 rpm, for 30 min at 4℃, the supernatant was removed and stored at -80℃ until use. The amount of protein in each sample was measured using a protein kit (BCA; Pierce, Rockford, IL, USA).
The protein homogenates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene difluoride membranes. The blots were immunoreacted with anti-cleaved caspase-3 antiserum (Cell Signaling Technology, Beverly, MA, USA) or anti-IL-6 antibody (Abcam, Tokyo, Japan). β-Actin (Sigma-Aldrich, St. Louis, MO, USA) was used as a loading control. The protein bands were visualized using a chemiluminescence detection system (Super-Signal West Pico; Pierce).
The rat pups were transcardially perfused with 0.1 M PBS containing 4% paraformaldehyde (PFA), and the brain tissues were removed and immersed in 4% PFA in PBS, for overnight post-fixation at 4℃. The samples were histologically analyzed as described previously [11]. Briefly, the brain tissues were paraffin-embedded and cut into 5-µm-thick sections. The sections were dewaxed, hydrated and subjected to antigen retrieval by autoclaving. Endogenous peroxidase activity and nonspecific staining were then blocked, and the sections were incubated with anti-cleaved caspase-3 antiserum (Cell Signaling Technology), diluted 1 : 300 in antibody diluent (Dako, Glostrup, Denmark) overnight in a humidified chamber at 4℃. Subsequently, the sections were incubated with peroxidase-conjugated secondary antibody (EnVision+ system; Dako), and then reacted with 3,3'-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. Finally, the sections were counterstained with hematoxylin.
Statistical analysis was performed using the Excel statistical software package (Excel-statistics 2010; Social Survey Research Information Co., Ltd., Tokyo, Japan). One-way analysis of variance followed by Newman-Keuls post hoc test was used for comparisons of data among groups. P value of < 0.05 was considered statistically significant. Values of P < 0.05 were considered to indicate statistical significance. Values are presented as means ± SD.
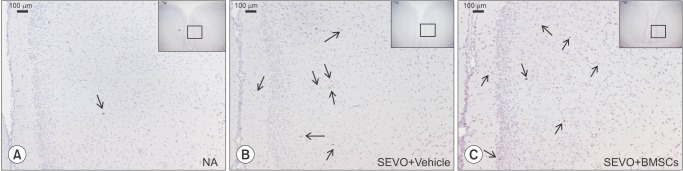
Immediately following the 6 h period of exposure to sevoflurane, the BMSC and vehicle treatment groups were assessed for proinflammatory cytokine IL-6, and activated (cleaved) caspase-3 levels, by western blotting. Analysis of the cleaved caspase-3 bands revealed that levels of activated caspase-3 were elevated in the vehicle group compared with the mock anesthesia group (P < 0.05), indicating that a single exposure to sevoflurane at subclinical concentrations can induce neuronal apoptosis. BMSC treatment did not suppress apoptosis induced by sevoflurane exposure (compared with the vehicle group, P < 0.05) (Fig. 1A). The vehicle group had higher proinflammatory cytokine IL-6 protein levels compared with the mock anesthesia group (P < 0.05), indicating that sevoflurane exposure induces IL-6 expression. BMSC treatment suppressed this sevoflurane-induced increase in IL-6 expression, indicating that these cells can inhibit neuroinflammation induced by sevoflurane exposure (vehicle group vs. BMSC group, P < 0.05) (Fig. 1B). Immunostaining for cleaved caspase-3 confirmed that there was no significant difference in cleaved caspase-3 levels between the BMSC and vehicle groups (Fig. 2). The number of caspase-3-positive cells was not significantly reduced by BMSC treatment.
In this study, for the first time, we examined the therapeutic potential of intravenous administration of BMSCs, for amelioration of sevoflurane-induced neurotoxicity, in the developing brain. We found that the administration of BMSCs reduced expression of the proinflammatory cytokine IL-6, while not affecting caspase-3 activation, following a 6 h period of sevoflurane exposure. The brain is highly susceptible to injury during the developmental period of rapid brain growth, including injury caused by sevoflurane exposure [91011]. Recent studies have shown that anesthesia with 3% sevoflurane, for 2 h daily for 3 d in neonatal mice, induces neuroinflammation and cognitive impairment in adulthood [9], and further that anesthesia with 2% sevoflurane, for 2 h in pregnant mice at gestational day 14, increases IL-6 levels and induces caspase-3 activation in the brain tissue of fetal mice [10].
To achieve zero mortality in the rat pups in this study, we determined that the optimal concentration of sevoflurane exposure was 2%, which is lower than that used in the studies mentioned above. We demonstrated that a single exposure to sevoflurane, at a subclinical concentration, induces neuroinflammation and neuronal apoptosis, which is consistent with recent studies [9101113]. Although hypoxemia, hypercapnia and hypoglycemia can potentially contribute to neuronal apoptosis during sevoflurane exposure, we did not perform blood gas or cerebral blood flow analysis, because numerous recent studies have shown that sevoflurane exposure does not impact these parameters [12311].
It has been reported that apoptosis precipitated by sevoflurane exposure in the developing brain is the main cause of cognitive impairment in adulthood. The majority of studies on sevoflurane-induced neurotoxicity during brain development have examined the effect of treatment strategies for apoptosis, followed by behavioral studies in adulthood [101315]. In the present study, we did not perform behavioral analysis to assess the therapeutic benefits of BMSC treatment, because there was no significant reduction in cleaved caspase-3 levels in the BMSC treatment group compared with the vehicle treatment group.
In recent years stem cell administration has been shown to possess substantial therapeutic potential, particularly in studies of neuroregeneration [171819]. Recent research has shown that BMSCs can alleviate neurological deficits, and promote recovery of the central nervous system, in Parkinson's disease, brain trauma, spinal cord injury and multiple sclerosis [162223]. BMSCs can pass through the blood-brain barrier, migrate to the brain and participate in neurogenesis and synaptic plasticity, resulting in functional recovery following brain damage [19]. During cerebral infarction, BMSCs acquire neural phenotypes and are able to produce neurotrophic factors and inhibit apoptosis [18].
We performed several pilot studies to determine the ideal dose of BMSCs. We found that a high dose of BMSCs (50 µl of 2 × 107 cells/ml) resulted in high mortality due to pulmonary infarction. As a result, we selected a smaller dose (50 µl of 1 × 107 cells/ml) [18]. Komatsu et al. [24] reported that brain regeneration occurs over a time course of days to weeks following brain damage. In the present study, we evaluated the therapeutic effects of BMSC administration only immediately following sevoflurane anesthesia. In future studies, we will examine the therapeutic effects of these cells over a more extended time frame.
In summary, we investigated, for the first time, the therapeutic potential of BMSCs for sevoflurane neurotoxicity in the neonatal period. Intravenous administration of BMSCs reduced proinflammatory cytokine IL-6 expression, but failed to attenuate apoptosis induced by sevoflurane exposure.
Acknowledgments
We wish to thank Drs. Tokujiro Uchida and Hiroyoki Ito (Department of Anesthesiology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan) for their generous gift of the BMSCs used in this study.
References
1. Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003; 23:876–882. PMID: 12574416.

2. Fredriksson A, Pontén E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007; 107:427–436. PMID: 17721245.
3. Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010; 112:834–841. PMID: 20234312.

4. Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, et al. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011; 33:592–597. PMID: 21708249.

5. Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration: determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci. 2010; 1199:43–51. PMID: 20633108.
6. Jevtovic-Todorovic V, Boscolo A, Sanchez V, Lunardi N. Anesthesia-induced developmental neurodegeneration: the role of neuronal organelles. Front Neurol. 2012; 3:141. PMID: 23087668.

7. Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006; 11:1603–1615. PMID: 16738805.

8. Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005; 135:815–827. PMID: 16154281.

9. Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013; 118:502–515. PMID: 23314110.

10. Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, et al. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology. 2013; 118:516–526. PMID: 23314109.

11. Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009; 110:628–637. PMID: 19212262.

12. Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010; 112:567–575. PMID: 20124973.

13. Yonamine R, Satoh Y, Kodama M, Araki Y, Kazama T. Coadministration of hydrogen gas as part of the carrier gas mixture suppresses neuronal apoptosis and subsequent behavioral deficits caused by neonatal exposure to sevoflurane in mice. Anesthesiology. 2013; 118:105–113. PMID: 23221861.

14. Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007; 106:746–753. PMID: 17413912.

15. Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012; 117:791–800. PMID: 22854980.

16. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005; 57:874–882. PMID: 15929052.

17. Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002; 1:92–100. PMID: 12849513.

18. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003; 73:778–786. PMID: 12949903.

19. Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A, et al. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant. 2008; 16:993–1005. PMID: 18351015.

20. Chen JR, Cheng GY, Sheu CC, Tseng GF, Wang TJ, Huang YS. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat. 2008; 213:249–258. PMID: 18647194.

22. Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells. 2013; 2:22–38. PMID: 23671814.
23. Zhou S. From bone to brain: human skeletal stem cell therapy for stroke. Cent Nerv Syst Agents Med Chem. 2011; 11:157–163. PMID: 21521166.

24. Komatsu K, Honmou O, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res. 2010; 1334:84–92. PMID: 20382136.

25. Zhou ZW, Shu Y, Li M, Guo X, Pac-Soo C, Maze M, et al. The glutaminergic, GABAergic, dopaminergic but not cholinergic neurons are susceptible to anaesthesia-induced cell death in the rat developing brain. Neuroscience. 2011; 174:64–70. PMID: 21056635.

Fig. 1
Membrane fractions were assessed for expression of IL-6 and cleaved caspase-3 in the BMSC and vehicle treatment groups, immediately subsequent to the 6 h period of sevoflurane exposure, by western blot analysis. The bars graphically represent the quantification of western blots normalized to β-actin content. (A) Following sevoflurane exposure, cleaved caspase-3 levels were increased in the vehicle group compared with the mock anesthesia group (P < 0.05), while there was no statistically significant difference between the BMSC and vehicle groups (P = 0.063). (B) Proinflammatory cytokine IL-6 expression was elevated in the vehicle group following sevoflurane exposure compared with the mock anesthesia group (P < 0.05). BMSC treatment reduced IL-6 expression following sevoflurane exposure compared with the vehicle group (P < 0.05). *P < 0.05; n = 7 animals in the vehicle and BMSC groups; n = 4 animals in the mock anesthesia group. BMSC: bone marrow stromal cell, NA (no anesthesia): mock anesthesia group.
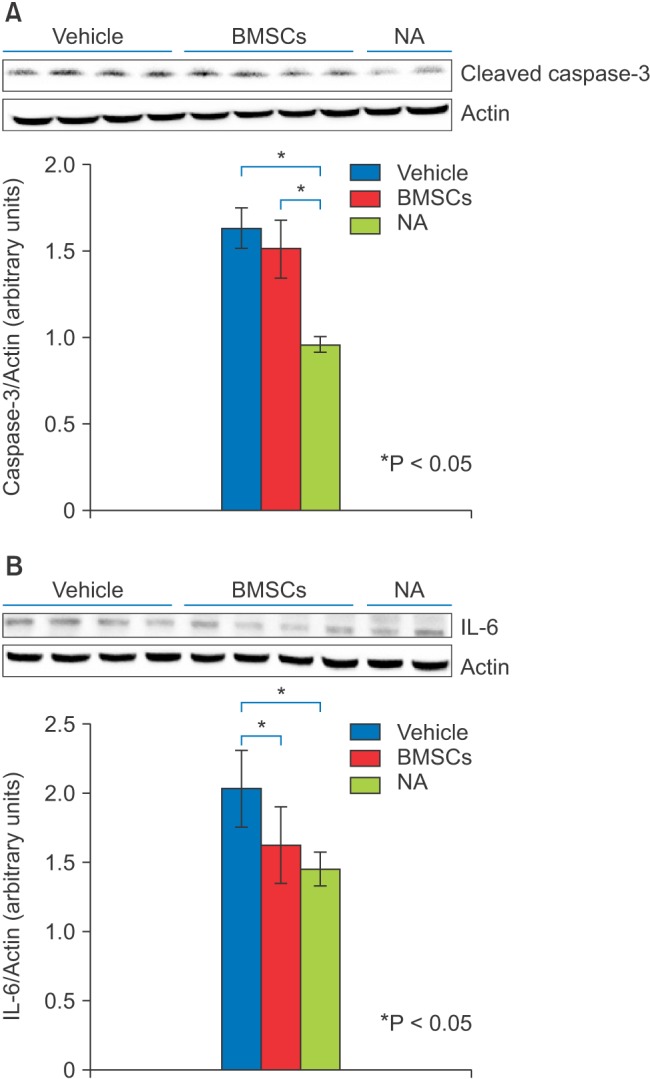
Fig. 2
Immunohistochemistry for activated caspase-3 following 6 h of sevoflurane exposure. Immunohistochemistry was performed on coronal sections (bregma -1.94 mm) as described in Zhou et al. [25]. Black arrows represent caspase-3-positive cells, indicating apoptosis. (A) Activated caspase-3 in the retrosplenial cortex of the mock anesthesia group. (B) Activated caspase-3 in the retrosplenial cortex of the vehicle group. (C) Activated caspase-3 in the retrosplenial cortex of the BMSC treatment group. Scale bars: 100 µm; ×10 magnification, BMSC: bone marrow stromal cell.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download