Abstract
The field of functional neurosurgery has expanded in last decade to include newer indications, new devices, and new methods. This advancement has challenged anesthesia providers to adapt to these new requirements. This review aims to discuss the nuances and practical issues that are faced while administering anesthesia for deep brain stimulation surgery.
Deep brain stimulator (DBS) placement is a minimally invasive procedure that involves placement of electrodes into deep brain structures using microelectrode recordings and macrostimulation findings [1]. These electrodes are then connected to an implantable pulse generator (IPG), which is usually placed in the subclavicular region [1]. DBS is an effective treatment modality for a variety of movement disorders such as Parkinson disease, essential tremor, dystonia [123] and has expanded as a method of treatment for other disorders such as chronic pain, and other cognitive disorders including Alzheimer's disease (AD) and Huntington's chorea [3456]. There are four main components to the deep brain stimulator which include: (1) the intracranial electrodes that are inserted surgically into the deep brain gray matter (2) an anchoring system (plastic ring) that fixes the electrode, (3) a single or double channel IPG and (4) an extension cable that connects the DBS electrodes to the pulse generator which is implanted in the chest wall or abdomen [78]. This procedure can be performed in a single surgical day or can be staged, with the connection of the pulse generator and electrodes done on another day. The aim of this review article is to discuss the nuances and practical issues that are faced while administering anesthesia during DBS surgery.
While evaluating a patient for DBS surgery, it is important to weigh the overall benefits to the risk associated with the surgery. Careful patient selection by both the surgical and anesthesia teams is essential. It is critical that the anesthesia provider evaluates each patient's co-morbidities as well as the patient's primary disease specific considerations. It is also important to evaluate each patient for anesthetic considerations for the procedure.
In addition to anesthesia, patients should be evaluated in terms of their family and social support. It is crucial for the patients to understand the risks and demands associated with different stages of surgery and to be able to comply with them. Patients and their family members should be educated regarding the immediate post-operative outcomes, complications, reasonable long term outcomes and peri-operative medications management. The need for multiple programming sessions and medications adjustment to achieve optimum clinical outcome should also be informed well in advance so as to have cooperation of the patient and their family. All the queries, doubts and expectations should also be resolved prior to surgery to relieve the anxiety and stress associated with an awake surgical procedure.
DBS surgery is most likely to benefit the appendicular symptoms as compared to axial symptoms such as posture, gait, and speech. Patients with severe tremors, bradykinesia, rigidity, freezing, dystonia (in OFF medication state), severe disabling dyskinesia with medications and disabling motor fluctuations in ON-OFF medication, are the most likely surgical candidates. The patient's response to levodopa with improvement in UPDRS-III score is the most important predictor of good surgical outcome following DBS surgery [3].
In general, patients with either postural, intention/action or rest tremors involving the distal extremities can be controlled with surgery. In contrast, the more proximal tremors are the most difficult to treat surgically [91011]. Head, neck and lower extremity tremors are often difficult to treat and require bilateral DBS implantation surgeries as compared to upper extremity tremors.
Patients with dystonia which is refractory to all the medication sand Botulinum toxin injections can be benefitted with DBS implantation surgery. Cases with primary generalized dystonia [12131415] as well as patients with idiopathic cervical dystonia can obtain the best motor control with bilateral globus pallidus internus (GPi) DBS. Juvenile-onset idiopathic dystonia with age of onset greater than five years and without multiple orthopedic deformities also has a good response to surgery.
Neuropsychological assessment is recommended and should include assessment of cognitive functions, mood disorders including other neuropsychiatric symptoms, family support, and realistic expectations [1617]. Patients with severe dementia or other neuropsychiatric symptoms should be deferred from DBS surgery and underlying condition should be addressed first. However, patients with mild cognitive dysfunction or frontal lobe dysexecutive syndrome may still undergo surgery but should receive extra counseling along with their family regarding the potential for increased risk of cognitive impairment and confusion post-surgery [161718]. Neuropsychiatric disorders such as anxiety, major depression, and mania must be identified and medically optimized preoperatively and an intra-operative anxiety counselor can also be arranged. DBS surgery in patients with psychotic disorders or severe personality disorders should be avoided.
Cardiac, pulmonary, and systemic conditions such as hypertension, diabetes and cancer should be stabilized prior to DBS surgery. Patients who are on chronic anti-platelet or Coumadin therapy must be able to tolerate complete withdrawal from these medications prior to surgery. Consultation with other medical specialists (e.g., cardiologists, pulmonologist, hematologist, nephrologist, and urologist) may be required in some patients prior to surgery.
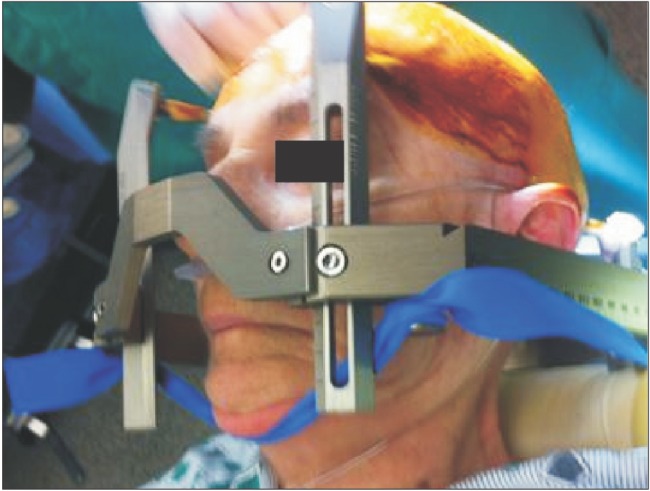
Airway management of patients undergoing DBS placement can be challenging yet an important task. Having the operating room table placed 180 degrees away from the anesthesia provider decreases airway accessibility. If a stereotactic frame is placed, the standard adult anesthesia circuit face mask cannot be utilized. In an emergency airway situation, management involves either the use of a pediatric face mask or laryngeal mask airway or removal of the frame. For this reason, any patient undergoing sedation for all or part of the procedure should have an advanced airway management plan in place [1]. Patients with co-morbidities such as obstructive sleep apnea, psychiatric disorders, or chronic pain will require special considerations and management. In Fig. 1, a tourniquet is used to aid the anesthesia provider in a hands free chin-lift to prevent airway obstruction. Patients with underlying Parkinson's disease or Dystonia are at higher risk for aspiration pneumonia and laryngospasm due to laryngo-pharyngeal dysfunction and dystonia [1]. Khatib et al. [19] reported an incidence of 1.60% (n = 258) of airway complications that include airway obstruction, prolonged intubation, postoperative acute respiratory distress syndrome, nosocomial pneumonia, or aspiration during DBS implantation surgery [19]. Similarly, Venkatraghavan et al. [20] experienced an incidence of 1.1% (n = 172) complications of airway obstruction and respiratory distress following DBS surgery [20].
MER are done by the neurophysiology team in order to detect and amplify individual neuronal activity at three primary target sites; subthalamic nuclei (STN), GPi, and the ventralis intermedius nucleus of the thalamus (Vim) [1]. Different anesthetic agents can decrease MER by lowering or eliminating spontaneous neuronal firing [21], and suppress motor signs such tremors and rigidity [22]. Since anesthetic drugs work differently on each region of the brain, the extent to which drugs influence MER are unknown [23].
Propofol is a common and frequently used anesthetic drug for sedation in deep brain stimulator cases due to its rapid onset of anesthesia and its rapid reversal [24]. In diseases such as dystonia or Parkinson's disease, propofol has been shown to cause differences in neuronal activity patterns [252627]. MER can be extremely difficult with the use of propofol due to the drug's GABA receptor-mediated sensitivity in subcortical areas of the brain [212728]. Intra-operative testing can be hindered or misinterpreted by the ability of propofol to suppress tremors [2729], and induce dyskinesias [3031]. Use of propofol has been associated with sneezing, which can increase intracranial pressure and lead to intracranial hemorrhage [73233]. Although, propofol can interfere with MER, Raz et al. [34] reported that it can be used safely throughout the placement of the stereotactic frame, skin incision, burr hole and the electrical activity returned to baseline after 9.3 ± 4.0 minutes (mean ± 2SDs, n = 24) of stopping the propofol prior to mapping [34].
Dexmedetomidine is more recently seen as the drug of choice for sedation in DBS procedures, being used alone or in conjunction with Propofol. Dexmedetomidine is an alpha-2-adrenergic agonist that causes sedation by acting on the subcortical areas, resembling natural sleep without causing respiratory depression [35]. In addition to its analgesic properties, and ability to decrease arterial blood pressure, it does not affect MER since it has a non-GABA-mediated mechanism of action [36].
Although, the use of a conscious sedation technique with both propofol and dexmedetomidine is preferred, individuals that cannot tolerate an awake technique such as pediatric patients, patients with psychiatric disorders, patients with extreme confusion, claustrophobia, severe anxiety with associated hypertension, and those with extreme pain due to dystonia may require a general anesthesia. The utility of scalp nerve blocks with local anesthetics have been described in various neurosurgical procedures [37]. A combination of scalp blocks and opioids continuous infusion (fentanyl and propofol) can also be used for maintaining conscious sedation in patients undergoing DBS surgery for PD without interfering with the MER [38].
Chen et al. [2] showed that a general anesthetic technique with the use of desflurane was an acceptable alternative anesthetic approach, with comparable motor outcomes to the conscious sedation technique in Parkinson's patients undergoing DBS implantation surgery. However, they found statistically significant deterioration of cognitive function in the general anesthesia group (P = 0.017) [2].
Arterial hypertension during surgery has been associated with intracranial bleeding [7]. This complication can be controlled through a pre- and intraoperative antihypertensive therapy. Adequate pain control, patient positioning, thermal control, and prevention of bladder distension with the use of a Foley catheter as well as avoidance of excessive fluid administration help to control the blood pressure during surgery [7]. Khatib et al. [19] reported a 2.8% incidence of intracranial hemorrhage (n = 258) and a cardiovascular complication rate of 0.40%, which included new onset angina, congestive heart failure and systemic arterial hypertension in patients who underwent DBS implantation surgery [19]. Intracranial hemorrhage due to uncontrolled hypertension during surgery is a devastating complication that can result in permanent neurological deficit [39]. To minimize the risks of intracranial hemorrhage, the anesthesia providers should screen patients for preoperative uncontrolled hypertension, coagulopathy, or recent use of antiplatelet medications [7]. Should bleeding occur intra-operatively, immediate airway stabilization and urgent brain imaging (CT or MRI) must be performed [7].
During the second stage, the DBS lead is connected to an extension wire which is tunneled from the scalp to the subclavicular region and is connected to an IPG [1]. This is typically performed anywhere from two days to three weeks after the initial procedure. This part of the procedure can be done under general anesthesia since microelectrode recordings are not required. Like with any case of general anesthesia, the patients' underlying pathology, co-morbidities, medications and risk factors for a difficult airway or aspiration should be taken into consideration.
When using electrocautery during the second stage or any subsequent surgery in a patient who has a DBS implantable device, it is important to turn off the pulse generator to avoid thermal injury to the brain tissue, reprogramming or damage to the device and its leads [4041]. During surgery, bipolar electrocoagulation should be used to reduce electromagnetic interference, and the device should be interrogated postoperatively [42]. DBS programming is typically done 4 weeks after the 1st stage of surgery to avoid the "microlesion" effect, in which possible edema formed around the implanted electrode causes improvement of patient symptoms and thus impairs the ability to detect stimulation-induced benefits [15].
Although, DBS placement and research have been done for years in adults, research pertaining to children is recent and very limited. This being said, Sebeo et al. [24] safely performed DBS implantation surgery in 28 pediatric patients (ages 7-17) with dystonia using combined propofol and dexmedetomidine sedation technique [24]. Their only major complication was a case of venous air embolism following the burr-hole placement. The procedure was aborted, and safely performed a month later [24]. The 3.6% incidence rate reported by the authors is similar to the average reported rate for this procedure [43]. Even though, the sedation technique can be applied successfully in the pediatric population, a careful patient selection should be performed.
Successful placement of a DBS device have been described in a 5 year-old child with dystonic storm under general anesthesia [44]. Due to tense masseter muscles and nasopharyngeal dystonia, such patients are likely to present with a difficult airway requiring special techniques including fiberoptic intubation [44].
AD belongs to the group of neurodegenerative disorders, dementia of Alzheimer's disease being the most common form of dementia. It is associated with progressive cognitive impairment encountering short term and long term memory loss [45]. General anesthetics may worsen the course of AD, and the detrimental effects are partially explained by suppression of acetylcholine (Ach) release in the brain [46]. This detrimental effects of anesthetic drugs are more pronounced with the use of volatile inhalation agents such as desflurane and sevoflurane [474849]. Caution should be used when performing a general anesthesia for DBS implantation surgery in patients with AD, so as not to further decrease their cognitive function or cause delirium in a patient with an already compromised central cholinergic neuronal transmission [5051]. The safety threshold of propofol is higher [51], possibly making it a safer alternative for a cooperative AD patient.
Dr. George Williams [52] from the University of Texas-Medical School had an elderly male presenting with conductive hearing loss in the pre-operative area for an urgent procedure [52]. This gentleman was unable to respond to any auditory instructions unless they were given by the familiar voice of his wife. Dr. Williams, with permission, recorded the wife's voice giving simple commands such as "wiggle your toes" and "take a deep breath" on his I-phone [52]. Dr. Williams then placed his phone by the patient's dominate ear in which the patient responded only to commands that were given by the familiar voice of his wife [52]. Many times individuals can only follow the command of the voices of their caregivers. The use of technological advancements allow for the pre-recording of familiar voices in order to have the patient be able to communicate and participate in their procedure and MER testing.
Special consideration needs to be taken with patients that have pacemakers. In past, deep brain stimulator was contraindicated in patients with cardiac pacemakers because of their possible negative interaction with each other [7]. Ozben et al. [53] have proven that a bipolar configuration of DBS and cardiac pacemakers are less prone to interaction and can be used together [5354]. Pre-operatively, cardiac pacemakers should be interrogated and placed in a bipolar mode for sensing and stimulation to minimize interference [55]. MRI testing is contraindicated with most pacemakers so a stereotactic CT should be used for imaging [55]. Pulse generators should be implanted as far away from a cardiac pacemakers as possible to minimize the possibilities of interference, and when initially programing the DBS, close observation of the ECG should be observed for interference [56].
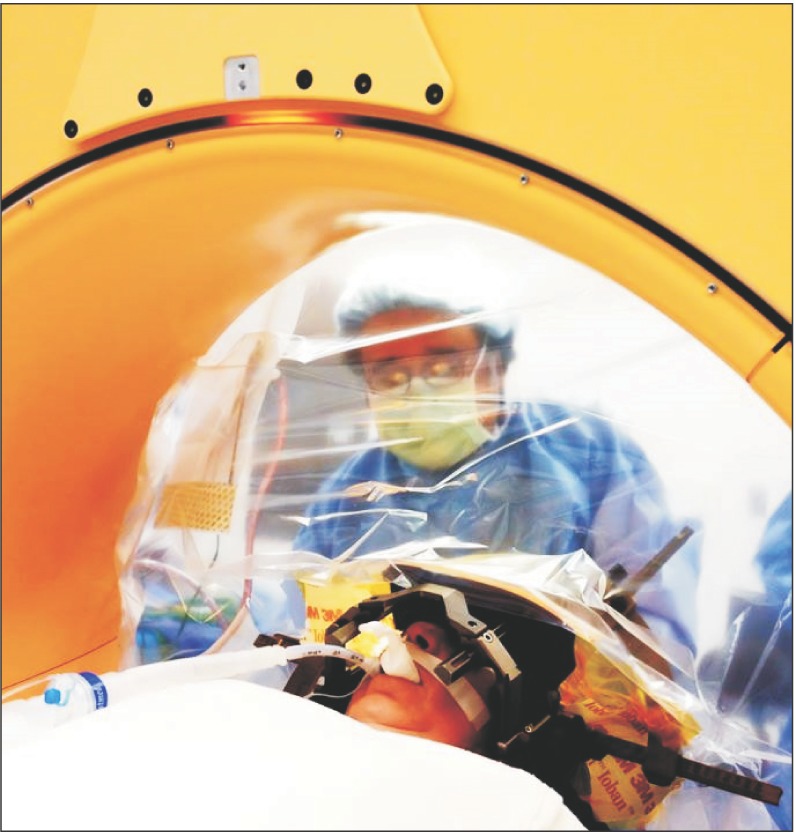
The Medtronic O-arm is a portable CT scanner that can be positioned around the patient's head throughout the procedure to provide intraoperative 3-dimensional confirmation of electrode tip locations, see Fig. 2 [57]. With this technique the patient can be placed under general anesthesia with an endotracheal tube. Upon arrival to the operating room the patient is intubated and, after securing the airway, the stereotactic frame may be placed. Like the awake technique, it is important to maintain tight blood pressure control during the procedure as well as during emergency in order to prevent intracranial bleeding.
Venous air embolism (VAE) is a complication that can occur at any time during the placement of a DBS. The complication can occur in both the supine, semi-sitting positions, and sitting position. Two major contributors of VAE are partial airway obstruction due to sedation and hypovolemia from perioperative fasting [4]. The most common initial symptom in an awake patient is coughing or sighing followed by tachypnea, hypoxemia, chest discomfort, tachycardia and hypotension [43]. In order to prevent this complication, the anesthesia provider should limit the extent of head elevation and warrant adequate hydration before the procedure [43]. If a VAE is suspected, the patient should be placed in Trendelenburg position, the surgical site should be irrigated, bone wax applied to any exposed edges, and any open vessels should be cauterized [43]. According to Palmon et al. [58], air aspiration can be attempted through a central line and rapid fluid administration and inotrope support may be used to maintain adequate perfusion and support the patients' blood pressure.
Seizures are another complication that can occur during DBS and should be managed with benzodiazepines or propofol for their termination [7]. Venkatraghavan et al. [20] reported a seizure incidence of 4.5% (n = 172) [20], while Khatib et al. [19] described an incidence of 0.80% among 258 patients [19]. Anesthesia providers should also be aware of the possibility of an akinetic crisis which is typically found in patients with severe Parkinson's disease, in which the patient is alert and conscious, but is unable to communicate [59].
PD patients discontinue their routine PD medications for at least 12 hours before surgery to aid in microelectrode recording (MER) and macro-stimulation during surgery to confirm the proper DBS lead placement [15]. Following completion of a DBS surgery, which usually lasts anywhere between 4 to 8 hours, the patients need to be given the medications as soon as possible. Delay in restarting the medication can result in worsening of PD symptoms and trigger severe dyskinesias and dystonias. Sudden or abrupt alteration in the dosage of Anti-PD medications during the post-operative period can lead to devastating complications including fatal Neuroleptic malignant syndrome or Parkinson-hyperpyrexia syndrome (NMS/PHS) [60]. Therefore patients should be started on their routine Anti-PD medications as soon as possible after DBS surgery.
Placement of DBS in STN itself exerts an anti-Parkinsonian effect immediately after surgery. This can act synergistically with the anti-PD medications and can produce severe peak-dose dyskinesias. It is important to watch the patient closely and titrate the preoperative anti-PD medications in patients who have a significant micro-subthalamotomy effect [6162].
Transient confusion is the most common neuropsychiatric side effect in the immediate postoperative period following STN DBS with an incidence ranging between 1 and 36% [6364]. Presence of clinically significant neurocognitive deficits prior to surgery is associated with increased confusion following DBS surgery [63]. Eyelid apraxia is also a commonly seen transient side effect of bilateral STN DBS [64]. This is transient and does not need any specific treatment but definitely affects the neurological assessment in the post-operative period.
Any medications that have an anti-dopaminergic effect like Haloperidol and metoclopramide can worsen PD symptoms and cause acute Parkinsonian crisis. The best anti-nausea medication for PD patients in the post-operative period is ondansetron. Ativan in small doses also works very well for confusion.
DBS is a safe, effective and well established treatment for movement disorders and epilepsy, and is being explored for the treatment of psychiatric disorders. With the rapid expansion of the horizons of functional neurosurgery, it is prudent for the anesthesiologist to be aware of the intricate details and the requirements of such procedures. Moreover, the extremes of age groups on which such procedures are performed add to the risks and complications associated with both the surgical procedure and the anesthesia. Therefore, appropriate understanding of the preoperative evaluation, intraoperative assessment and postoperative management in such patients undergoing deep brain stimulator implantation surgery results in outstanding outcomes.
References
1. Venkatraghavan L, Luciano M, Manninen P. Review article: anesthetic management of patients undergoing deep brain stimulator insertion. Anesth Analg. 2010; 110:1138–1145. PMID: 20142347.
2. Chen SY, Tsai ST, Lin SH, Chen TY, Hung HY, Lee CW, et al. Subthalamic deep brain stimulation in Parkinson's disease under different anesthetic modalities: a comparative cohort study. Stereotact Funct Neurosurg. 2011; 89:372–380. PMID: 22104439.

3. Rezai AR, Sharma M. Deep Brain Stimulation (DBS) : Current and Emerging Applications. Jpn J Neurosurg (Tokyo). 2014; 23:648–660.
4. Lozano AM, Hamani C. The future of deep brain stimulation. J Clin Neurophysiol. 2004; 21:68–69. PMID: 15097295.

5. Zibly Z, Shaw A, Harnof S, Sharma M, Graves C, Deogaonkar M, et al. Modulation of mind: therapeutic neuromodulation for cognitive disability. J Clin Neurosci. 2014; 21:1473–1477. PMID: 24882563.

6. Sharma M, Deogaonkar M. Deep brain stimulation in Huntington's disease: assessment of potential targets. J Clin Neurosci. 2015; 22:812–817. PMID: 25698541.

7. Poon CC, Irwin MG. Anaesthesia for deep brain stimulation and in patients with implanted neurostimulator devices. Br J Anaesth. 2009; 103:152–165. PMID: 19556271.

8. Sharma M, Rhiew R, Deogaonkar M, Rezai A, Boulis N. Accuracy and precision of targeting using frameless stereotactic system in deep brain stimulator implantation surgery. Neurol India. 2014; 62:503–509. PMID: 25387619.

9. Berk C, Carr J, Sinden M, Martzke J, Honey CR. Thalamic deep brain stimulation for the treatment of tremor due to multiple sclerosis: a prospective study of tremor and quality of life. J Neurosurg. 2002; 97:815–820. PMID: 12405368.

10. Deuschl G, Bain P. Deep brain stimulation for tremor [correction of trauma]: patient selection and evaluation. Mov Disord. 2002; 17(Suppl 3):S102–S111. PMID: 11948763.
11. Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, et al. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology. 2000; 55:114–116. PMID: 10891917.

12. Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V. Pallidal stimulation for dystonia. Neurosurgery. 2004; 55:1361–1368. PMID: 15574217.

13. Kupsch A, Klaffke S, Kuhn AA, Meissner W, Arnold G, Schneider GH, et al. The effects of frequency in pallidal deep brain stimulation for primary dystonia. J Neurol. 2003; 250:1201–1205. PMID: 14586602.

14. Lee JY, Deogaonkar M, Rezai A. Deep brain stimulation of globus pallidus internus for dystonia. Parkinsonism Relat Disord. 2007; 13:261–265. PMID: 17081796.

15. Rezai AR, Kopell BH, Gross RE, Vitek JL, Sharan AD, Limousin P, et al. Deep brain stimulation for Parkinson's disease: surgical issues. Mov Disord. 2006; 21(Suppl 14):S197–S218. PMID: 16810673.

16. Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson's disease: surgical technique and perioperative management. Mov Disord. 2006; 21(Suppl 14):S247–S258. PMID: 16810722.

17. Machado AG, Deogaonkar M, Cooper S. Deep brain stimulation for movement disorders: patient selection and technical options. Cleve Clin J Med. 2012; 79(Suppl 2):S19–S24. PMID: 22761265.
18. Rothlind JC, York MK, Carlson K, Luo P, Marks WJ Jr, Weaver FM, et al. Neuropsychological changes following deep brain stimulation surgery for Parkinson's disease: comparisons of treatment at pallidal and subthalamic targets versus best medical therapy. J Neurol Neurosurg Psychiatry. 2015; 86:622–629. PMID: 25185211.

19. Khatib R, Ebrahim Z, Rezai A, Cata JP, Boulis NM, John Doyle D, et al. Perioperative events during deep brain stimulation: the experience at cleveland clinic. J Neurosurg Anesthesiol. 2008; 20:36–40. PMID: 18157023.

20. Venkatraghavan L, Manninen P, Mak P, Lukitto K, Hodaie M, Lozano A. Anesthesia for functional neurosurgery: review of complications. J Neurosurg Anesthesiol. 2006; 18:64–67. PMID: 16369142.
21. Ruskin DN, Bergstrom DA, Kaneoke Y, Patel BN, Twery MJ, Walters JR. Multisecond oscillations in firing rate in the basal ganglia: robust modulation by dopamine receptor activation and anesthesia. J Neurophysiol. 1999; 81:2046–2055. PMID: 10322046.

22. Anderson BJ, Marks PV, Futter ME. Propofol--contrasting effects in movement disorders. Br J Neurosurg. 1994; 8:387–388. PMID: 7946034.

23. Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007; 107:202–212. PMID: 17667563.

24. Sebeo J, Deiner SG, Alterman RL, Osborn IP. Anesthesia for pediatric deep brain stimulation. Anesthesiol Res Pract. 2010; 2010:401419. PMID: 20814550.

25. Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: implications for models of dystonia. Ann Neurol. 2003; 53:480–488. PMID: 12666115.

26. Maltete D, Navarro S, Welter ML, Roche S, Bonnet AM, Houeto JL, et al. Subthalamic stimulation in Parkinson disease: with or without anesthesia? Arch Neurol. 2004; 61:390–392. PMID: 15023817.
27. Lettieri C, Rinaldo S, Devigili G, Pauletto G, Verriello L, Budai R, et al. Deep brain stimulation: Subthalamic nucleus electrophysiological activity in awake and anesthetized patients. Clin Neurophysiol. 2012; 123:2406–2413. PMID: 22683113.

28. Hutchison WD, Lozano AM. Microelectrode recordings in movement disorder surgery. In : Lozano AM, editor. Movement Disorder Surgery. Basel: Karger;2000. p. 103–117.
29. Bohmdorfer W, Schwarzinger P, Binder S, Sporn P. Temporary suppression of tremor by remifentanil in a patient with Parkinson's disease during cataract extraction under local anesthesia. Anaesthesist. 2003; 52:795–797. PMID: 14504805.
30. Deogaonkar A, Deogaonkar M, Lee JY, Ebrahim Z, Schubert A. Propofol-induced dyskinesias controlled with dexmedetomidine during deep brain stimulation surgery. Anesthesiology. 2006; 104:1337–1339. PMID: 16732105.

31. Krauss JK, Akeyson EW, Giam P, Jankovic J. Propofol-induced dyskinesias in Parkinson's disease. Anesth Analg. 1996; 83:420–422. PMID: 8694329.

32. Fabregas N, Rapado J, Gambus PL, Valero R, Carrero E, Salvador L, et al. Modeling of the sedative and airway obstruction effects of propofol in patients with Parkinson disease undergoing stereotactic surgery. Anesthesiology. 2002; 97:1378–1386. PMID: 12459662.
33. Tao J, Nunery W, Kresovsky S, Lister L, Mote T. Efficacy of fentanyl or alfentanil in suppressing reflex sneezing after propofol sedation and periocular injection. Ophthal Plast Reconstr Surg. 2008; 24:465–467.

34. Raz A, Eimerl D, Zaidel A, Bergman H, Israel Z. Propofol decreases neuronal population spiking activity in the subthalamic nucleus of Parkinsonian patients. Anesth Analg. 2010; 111:1285–1289. PMID: 20841416.

35. Rozet I. Anesthesia for functional neurosurgery: the role of dexmedetomidine. Curr Opin Anaesthesiol. 2008; 21:537–543. PMID: 18784476.

36. Rozet I, Muangman S, Vavilala MS, Lee LA, Souter MJ, Domino KJ, et al. Clinical experience with dexmedetomidine for implantation of deep brain stimulators in Parkinson's disease. Anesth Analg. 2006; 103:1224–1228. PMID: 17056959.

37. Chaki T, Sugino S, Janicki PK, Ishioka Y, Hatakeyama Y, Hayase T, et al. Efficacy and Safety of a Lidocaine and Ropivacaine Mixture for Scalp Nerve Block and Local Infiltration Anesthesia in Patients Undergoing Awake Craniotomy. J Neurosurg Anesthesiol. 2014; [Epub ahead of print].

38. Kim W, Song IH, Lim YH, Kim MR, Kim YE, Hwang JH, et al. Influence of propofol and fentanyl on deep brain stimulation of the subthalamic nucleus. J Korean Med Sci. 2014; 29:1278–1286. PMID: 25246748.

39. Binder DK, Rau GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005; 56:722–732. PMID: 15792511.

40. Milligan DJ, Milligan KR. Deep brain neuro-stimulators and anaesthesia. Anaesthesia. 2007; 62:852–853. PMID: 17635442.

41. Weaver J, Kim SJ, Lee MH, Torres A. Cutaneous electrosurgery in a patient with a deep brain stimulator. Dermatol Surg. 1999; 25:415–417. PMID: 10469084.

42. Martinelli PT, Schulze KE, Nelson BR. Mohs micrographic surgery in a patient with a deep brain stimulator: a review of the literature on implantable electrical devices. Dermatol Surg. 2004; 30:1021–1030. PMID: 15209793.

43. Hooper AK, Okun MS, Foote KD, Haq IU, Fernandez HH, Hegland D, et al. Venous air embolism in deep brain stimulation. Stereotact Funct Neurosurg. 2009; 87:25–30. PMID: 19039260.

44. Aydin S, Abuzayed B, Uysal S, Unver O, Uzan M, Mengi M, et al. Pallidal deep brain stimulation in a 5-year-old child with dystonic storm: case report. Turk Neurosurg. 2013; 23:125–128. PMID: 23344881.
45. Bourin M, Ripoll N, Dailly E. Nicotinic receptors and Alzheimer's disease. Curr Med Res Opin. 2003; 19:169–177. PMID: 12814128.

46. Ma J, Shen B, Stewart LS, Herrick IA, Leung LS. The septohippocampal system participates in general anesthesia. J Neurosci. 2002; 22:RC200. PMID: 11784812.

47. Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999; 22:273–280. PMID: 10354606.

48. Tassonyi E, Charpantier E, Muller D, Dumont L, Bertrand D. The role of nicotinic acetylcholine receptors in the mechanisms of anesthesia. Brain Res Bull. 2002; 57:133–150. PMID: 11849819.

49. Fodale V, Santamaria LB. Drugs of anesthesia, central nicotinic receptors and post-operative cognitive dysfunction. Acta Anaesthesiol Scand. 2003; 47:1180. PMID: 12969118.

50. Pratico C, Quattrone D, Lucanto T, Amato A, Penna O, Roscitano C, et al. Drugs of anesthesia acting on central cholinergic system may cause post-operative cognitive dysfunction and delirium. Med Hypotheses. 2005; 65:972–982. PMID: 16043305.
51. Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB. Alzheimer's disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth. 2006; 97:445–452. PMID: 16950812.

52. Williams G. Demented and hearing loss patient. J Neurosurg Anesthesiol. 2013; 25:355. PMID: 23632428.

53. Ozben B, Bilge AK, Yilmaz E, Adalet K. Implantation of a permanent pacemaker in a patient with severe Parkinson's disease and a preexisting bilateral deep brain stimulator. Int Heart J. 2006; 47:803–810. PMID: 17106151.

54. Romano M, Brusa S, Grieco A, Zucco F, Spinelli A, Allaria B. Efficacy and safety of permanent cardiac DDD pacing with contemporaneous double spinal cord stimulation. Pacing Clin Electrophysiol. 1998; 21:465–467. PMID: 9507551.

55. Senatus PB, McClelland S 3rd, Ferris AD, Ford B, Winfield LM, Pullman SL, et al. Implantation of bilateral deep brain stimulators in patients with Parkinson disease and preexisting cardiac pacemakers. Report of two cases. J Neurosurg. 2004; 101:1073–1077. PMID: 15597774.
56. Capelle HH, Simpson RK Jr, Kronenbuerger M, Michaelsen J, Tronnier V, Krauss JK. Long-term deep brain stimulation in elderly patients with cardiac pacemakers. J Neurosurg. 2005; 102:53–59. PMID: 15658096.

57. Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: technique and accuracy assessment. Neurosurgery. 2011; 68(1 Suppl Operative):114–124. PMID: 21206322.

58. Palmon SC, Moore LE, Lundberg J, Toung T. Venous air embolism: a review. J Clin Anesth. 1997; 9:251–257. PMID: 9172037.

59. Schulz U, Keh D, Barner C, Kaisers U, Boemke W. Bispectral index monitoring does not improve anesthesia performance in patients with movement disorders undergoing deep brain stimulating electrode implantation. Anesth Analg. 2007; 104:1481–1487. PMID: 17513646.

60. Urasaki E, Fukudome T, Hirose M, Nakane S, Matsuo H, Yamakawa Y. Neuroleptic malignant syndrome (parkinsonism-hyperpyrexia syndrome) after deep brain stimulation of the subthalamic nucleus. J Clin Neurosci. 2013; 20:740–741. PMID: 23465352.

61. Krack P, Fraix V, Mendes A, Benabid AL, Pollak P. Postoperative management of subthalamic nucleus stimulation for Parkinson's disease. Mov Disord. 2002; 17(Suppl 3):S188–S197. PMID: 11948776.

62. Nutt JG, Rufener SL, Carter JH, Anderson VC, Pahwa R, Hammerstad JP, et al. Interactions between deep brain stimulation and levodopa in Parkinson's disease. Neurology. 2001; 57:1835–1842. PMID: 11723273.

63. Pilitsis JG, Rezai AR, Boulis NM, Henderson JM, Busch RM, Kubu CS. A preliminary study of transient confusional states following bilateral subthalamic stimulation for Parkinson's disease. Stereotact Funct Neurosurg. 2005; 83:67–70. PMID: 15990470.

64. Rezai AR, Machado AG, Deogaonkar M, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurgery. 2008; 62(Suppl 2):809–838. PMID: 18596424.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download