Abstract
Background
Notification of sugammadex has been supplemented with a section on hemostasis, including a longer clotting time in the first minutes following injection, without any documented clinical consequences. The objective of this observational study was to analyze the effects of sugammadex administration on routine coagulation tests and bleeding in the clinical setting.
Methods
After Institutional Review Board approval, a prospective observational study was conducted between January and December 2011. Adult patients scheduled for laparotomies were analyzed in groups according to the type of reversal (without sugammadex versus 2 or 4 mg/kg sugammadex). There were no changes in our current clinical practice. Blood samples drawn from these patients were standardized at the same time and tested using the same daily calibrated machine. The endpoint was a comparison of the activated partial thromboplastin time (aPTT), prothrombin time (PT), hemoglobin (Hb) level and hematocrit (Ht), immediately before sugammadex administration (H0) and 1 h after neuromuscular block reversal (H1).
Results
One hundred and forty-two patients in three groups were included as follows: 11 in the "without sugammadex" group, 64 in the "2 mg/kg sugammadex" group and 67 in the "4 mg/kg sugammadex" group. Results did not differ significantly among the groups.
Conclusions
In this prospective observational study, the use of 2 and 4 mg/kg sugammadex was not associated with a longer clotting time or decreased hemoglobin concentrations. Future prospective investigations should study patients receiving 16 mg/kg sugammadex and/or with abnormal coagulation tests.
Neuromuscular blocking agents are used during anesthesia to facilitate tracheal intubation, artificial ventilation, and surgical procedures. Reversal agents are often administered to obtain a shorter recovery from a neuromuscular block and prevent postoperative residual paralysis [1]. Sugammadex reverses neuromuscular blockade by specific chemical encapsulation of nondepolarizing neuromuscular blocking drugs (rocuronium and vecuronium) [1,2,3,4]. The notification of this new molecule was supplemented with a section on hemostasis, notifying a longer clotting time in the first minutes following injection without any documented clinical consequences (< 30 minutes). In volunteers, doses of 4 mg/kg and 16 mg/kg sugammadex prolonged the activated partial thromboplastin time (aPTT) and prothrombin time (PT) by 17-22%. Based on a clinical database (n = 1,738) and on a specific study on 1,184 patients undergoing hip fracture or joint replacement surgery, there was no clinically relevant effect of 4 mg/kg sugammadex alone or in combination with anticoagulants on the incidence of peri- or post-operative bleeding complications. However, these observations have resulted in recommendations on the use of sugammadex in the presence of coagulation disorders (pharmacologically induced or not). The objective of this study was to analyze the effect of sugammadex on routine coagulation tests (aPTT and PT) and bleeding (hemoglobin [Hb] and hematocrit [Ht]) in a clinical setting.
Between January and December 2011, a prospective observational study was conducted at the Cancer Institute of Lorraine in Nancy (France) that enrolled patients undergoing open abdominal cancer surgery. Exclusion criteria were as follows: age < 18 years, surgery with hyperthermic intraperitoneal chemotherapy, perioperative transfusion, pre-existing coagulation disorders and anticoagulant or antiaggregant therapy. The study was performed according to the declaration of Helsinki. The Institutional Review Board committee was informed and accepted the study. There was no change in clinical practice and no randomization of the patients.
The reversal agent was administered at the end of the procedure after surgical hemostasis and wound closure, according to the train of four (TOF) count. Neuromuscular block reversal was at the discretion of the anesthesiologist in charge of the patient: 4 mg/kg sugammadex for a deep block (post tetanic count [PTC] > 1 and TOF < 2/4, 2 mg/kg sugammadex for a moderate block [TOF ≥ 2] or 40 µg/kg neostigmine for a moderate block [TOF ≥ 4]). Data collection included age, sex, height, weight, body mass index (calculated as weight/height2), American Society of Anesthesiologists (ASA) score, duration and type of surgery, esophagus temperature before and after neuromuscular block reversal, surgical reintervention for hemostasis or transfusion in the first 24 hours post procedure. Biological data for hemostasis (aPTT and PT) and bleeding (Hb and Ht) were collected at different times as usual in our practice: pre-operative anesthetic assessment (PO), immediately before neuromuscular block reversal (H0), 1 hour after neuromuscular block reversal (H1) and the day after surgery at 6 a.m. (H24). Blood samples were collected from a peripheral vein, on an arm or a leg without perfusion.
Three groups of patients were analyzed according to the type of reversal (without sugammadex, 2 mg/kg sugammadex or 4 mg/kg sugammadex). The primary endpoint of this study was comparison of the variations of aPTT, PT, Hb and Ht after the injection of sugammadex (H1-H0) among the groups. The same parameters were analyzed (H0-PO and H24-H1), respectively, at the perioperative dilution and at the first postoperative day.
Biological measurements were carried out in the same laboratory using the same analyzer at the University Hospital of Nancy. Blood samples were obtained using the ACT TPOCTS analyzer (Instrumentation Laboratory, Lexington, KY, USA) for a complete blood count and the ADVIA 2120 analyzer (Siemens, Paris, France) for hemostasis. Both analyzers were calibrated daily.
Statistical analyses were performed using the R software version 2.14 (R Foundation for Statistical Computing, Vienna, Austria). Data were summarized by dose groups. Summary statistics (mean ± standard deviation, median) were used to describe continuous variables, and frequency counts and percentages were used for categorical variables. Comparison between continuous variables was performed using the Kruskal-Wallis non-parametric analysis of variance test. Comparison between categorical data was performed using Fisher's exact test. A P value less than 0.05 was deemed to indicate statistical significance.
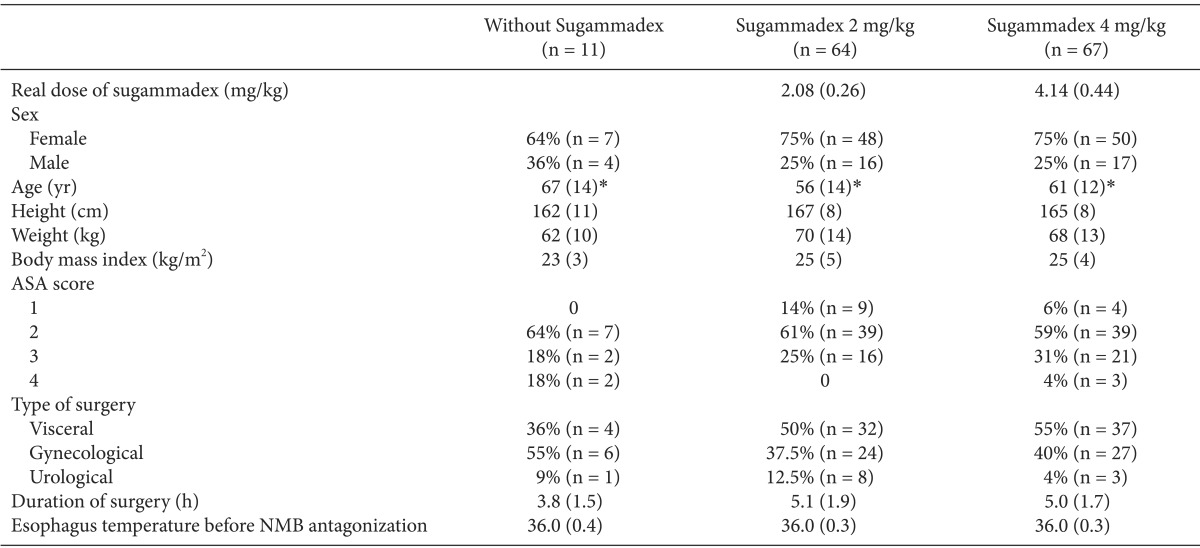
One hundred and forty-two patients were included in this prospective observational study: 11 in the "without sugammadex" group, 64 in the "2 mg/kg sugammadex" group and 67 in the "4 mg/kg sugammadex" group. Demographic, type of surgical procedure, and dose of sugammadex data are presented in Table 1. No difference was found in these data, with the exception of age. The types of surgery did not differ significantly among the three groups. The procedures were as follows: 51% underwent gastrointestinal surgery, 40% underwent gynecological procedures, and 9% underwent urological procedures. These procedures accounted for the different types of gynecological cancer surgery (colpohysterectomy with annexectomy and/or lymphadenectomy, oophorectomy), urologic surgery (nephrectomy, prostatectomy, cystectomy with or without reconstruction) or visceral surgery (bowel resection, liver resection, para-aortic lymphadenectomy). In this observational study, there was no injection of 16 mg/kg sugammadex. In the "without sugammadex" group, all 11 patients were reversed with neostigmine. All of the patients received an injection of enoxaparin (4,000 IU) 6 hours after the end of the surgery to prevent thrombotic events.
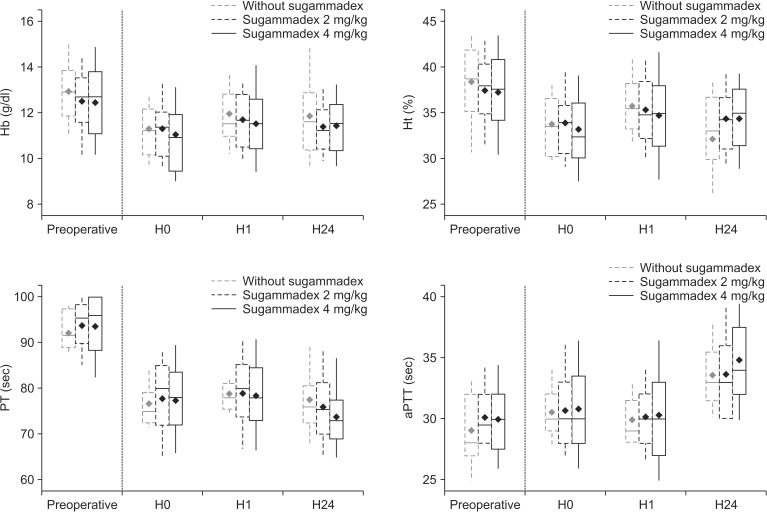
The three groups "without sugammadex" (n = 11), "2 mg/kg sugammadex" (n = 64) and "4 mg/kg sugammadex" (n = 67) were compared. The doses of 2 mg/kg and 4 mg/kg sugammadex did not prolong aPTT and PT during the first hour after administration (Fig. 1). Additionally, no difference was found regarding the hemostasis and bleeding data (Fig. 1). We did not find any surgical reinterventions for hemostasis or transfusion within the first 24 h post procedure.
The results of this prospective observational study did not demonstrate any significant difference in aPTT and PT after sugammadex at a dose of 2 or 4 mg/kg.
At our institution, the ratio of aPTT before the potential risk of surgical bleeding is 1.2. Subsequently, the normal value of aPTT is approximately 30 s, and the patient is considered at risk for eventual bleeding when the aPTT is above 36 s. In our study, 1 h after injection of sugammadex, the mean of the variation in the three groups did not exceed the values at risk for surgical bleeding. Regarding the other parameters, a value of PT below 70% could also be responsible for surgical bleeding. Similarly, 1 h after the injection of sugammadex, the average laboratory values in the three groups did not decrease below this level. To summarize, the coagulations were within the normal range with no risk of potential bleeding due to coagulation disorders.
Although our patients received a significant amount of intravenous fluids perioperatively because of the duration of surgery, the coagulation tests were not significantly altered. Concerning the central temperature, which could influence coagulation, there was no significant difference in temperature among the three groups. However, our study has some limitations. Regarding the additional information on sugammadex and hemostasis, only high doses (4 and 16 mg/kg) prolonged the aPTT and PT by 17-22%. In our study, there was no use of the larger recommended dose of 16 mg/kg. Interestingly, the number of patients receiving 4 mg/kg (n = 67) was much higher than that in our initial study (n = 20) [5]. It must be highlighted that, in our study, the "without sugammadex" group is small (n = 11) compared with the other groups because it is an observational study with no change in our clinical practice. The relative weakness of our study is that we did not explore the combination with anticoagulants or preexisting coagulation disorders, whereas recent recommendations on the use of sugammadex highlight a likely pharmacodynamic interaction (aPTT and PT prolongation) with vitamin K antagonists, unfractionated heparin, low-molecular-weight heparinoids, rivaroxaban and dabigatran. To date, routine coagulation tests may not detect a minimal interaction or an alteration of the coagulation. Standard coagulation point-of-care techniques such as thromboelastometry could provide more relevant information concerning the effect of sugammadex on the clotting time.
Regarding the mechanism by which sugammadex affects coagulation tests, data provided from the notification and previously published data are not sufficiently detailed to elucidate the potential contribution of sugammadex to the increase in PT or aPTT [5]. In the first study with volunteers, moderate changes in coagulation tests were observed; however, it was unclear whether some volunteers received sugammadex without a steroidal non-depolarizing muscle relaxant agent (NMBA). , as previously discussed, several factors may influence PT and aPTT during surgery (e.g., the use of plasma expanders [6] and central temperature [7,8,9]), and no information exists on a potential influence of plasma dilution. Hypothermia has been found to impair the coagulation cascade and to increase blood loss and the requirement for transfusion [7,8]. For example, a central temperature below 35℃ may prolong aPTT by 10% [9]. A more recent study demonstrated that sugammadex 4 mg/kg or 16 mg/kg for rocuronium-induced neuromuscular block reversal do not change anti-Xa activity or aPTT to a clinically meaningful extent following pretreatment with enoxaparin [10]. The conclusion of our study showed the same trend. There was no difference among our three groups 24 h after surgery despite the injection of enoxaparin 6 h after the end of the surgery.
In conclusion, in this observational prospective study, sugammadex at a dose of 2 or 4 mg/kg was not associated with a significant increase in clotting time or decreased postoperative hemoglobin concentrations. Further studies are needed to explore the interaction between sugammadex and the coagulation system and further prospective investigations should assess patients receiving higher doses of sugammadex.
References
1. Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010; 112:1013–1022. PMID: 20234315.

2. Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007; 104:575–581. PMID: 17312211.

3. Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005; 103:695–703. PMID: 16192761.

4. Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J Med Chem. 2002; 45:1806–1816. PMID: 11960492.

5. Raft J, Betala Belinga JF, Jurkolow G, Desandes E, Longrois D, Meistelman C. Clinical evaluation of post-surgical bleeding after a sugammadex injection. Ann Fr Anesth Reanim. 2011; 30:714–717. PMID: 21741200.
6. Reed RL 2nd, Johnston TD, Chen Y, Fischer RP. Hypertonic saline alters plasma clotting times and platelet aggregation. J Trauma. 1991; 31:8–14. PMID: 1986137.

7. Michelson AD, McGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994; 71:633–640. PMID: 7522354.

8. Reed RL 2nd, Johnson TD, Hudson JD, Fischer RP. The disparity between hypothermic coagulopathy and clotting studies. J Trauma. 1992; 33:465–470. PMID: 1404519.

9. Felfernig M, Blaicher A, Kettner SC, Felfernig D, Acimovic S, Kozek-Langenecker SA. Effects of temperature on partial thromboplastin time in heparinized plasma in vitro. Eur J Anaesthesiol. 2001; 18:467–470. PMID: 11437875.

10. De Kam PJ, Kruithof AC, van Lierop MJ, Moerland M, Dennie J, Troyer MD, et al. Lack of a clinically relevant effect of sugammadex on anti-Xa activity or activated partial thromboplastin time following pretreatment with either unfractionated or low-molecular-weight heparin in healthy subjects. Int J Clin Pharmacol Ther. 2014; 52:631–641. PMID: 24800921.
Fig. 1
Comparison of parameters before and after sugammadex. H0: control value (just before the neuromuscular block antagonization), H1: 1 hour after sugammadex administration, H24: the day after surgery at 6 a.m. (P > 0.05 for the comparison among different groups). Open boxes represent the 25th to 75th percentiles and contain the median (horizontal bar) and the mean (black diamond); vertical bars represent 10th and 90th percentiles. Hb: hemoglobin, Ht: hematocrit, PT: prothrombin time, aPTT: activated partial thromboplastin time.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download