Abstract
QT interval prolongation is associated with an increased risk of ventricular arrhythmia in various conditions. Cardiac electrophysiologic abnormalities including QT interval prolongation are well documented in patients with advanced liver cirrhosis. We report two cases of patients with QT interval prolongation on preoperative electrocardiography who exhibited repetitive ventricular arrhythmias with significant hemodynamic deterioration during liver transplantation. For the treatment and prevention of ventricular arrhythmias during the intraoperative period, we performed intravenous administration of lidocaine and isoproterenol, corrected imbalances of electrolytes including potassium and magnesium, and prepared a defibrillator. These cases emphasize that preoperative recognition of QT interval prolongation and adequate management to prevent fatal arrhythmias are important in patients undergoing liver transplantation.
Go to : 
QT interval prolongation is a manifestation on an electrocardiogram (ECG) arising from prolonged ventricular repolarization [1]. This electrophysiologic abnormality is caused not only by several congenital syndromes associated with inherited defects of cardiac ion channels but also by acquired conditions including electrolyte imbalance, subarachnoid hemorrhage, diabetic autonomic neuropathy, liver cirrhosis, and drugs which can slow ventricular repolarization [1,2]. The clinical considerations during anesthesia for patients with QT interval prolongation include the occurrence of serious arrhythmias such as torsades de pointes (TdP) provoked by various stimuli [3]. Since Day et al. [4] first reported significant QT interval prolongation in patients with alcoholic liver disease, several reports have provided evidence about the relationship between the prevalence of QT interval prolongation and the severity of liver cirrhosis. During liver transplantation in recipients with QT interval prolongation, the fatal arrhythmias can be triggered by surgical stress, electrolyte abnormalities, reperfusion injury, and various medications including anesthetics that influence the QT interval [5]. We herein report two cases of patients with QT interval prolongation on preoperative ECG who exhibited repetitive ventricular arrhythmias with significant hemodynamic deterioration during liver transplantation.
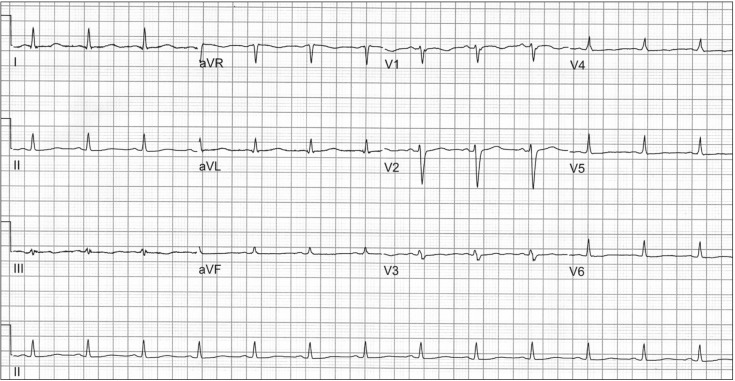
A 58-year-old, 175 cm, 64 kg male patient underwent liver transplantation due to liver cirrhosis caused by the hepatitis B virus, combined with hepatocellular carcinoma (HCC). The patient had a history of radiofrequency ablation for HCC and hemostasis for variceal bleeding and had taken terlipressin and entecavir. A preoperative ECG revealed normal sinus rhythm, a nonspecific T wave abnormality, and prolonged QT interval (QT/QTc 452/521) (Fig. 1), and preoperative transthoracic echocardiography (TTE) showed a left ventricular ejection fraction of 60%, a relaxation abnormality of the left ventricular filling pattern (E/E' = 13), and a right ventricular systolic pressure of 39 mmHg. After induction of anesthesia, the patient's hemodynamic variables were as follows: systemic blood pressure 109/59 mmHg, heart rate 69 bpm, central venous pressure 15 mmHg, and pulmonary arterial pressure 33/18 mmHg. The results of the initial electrolyte studies showed Na+ 136 mmol/L, K+ 3.2 mmol/L, Cl- 105 mmol/L, Ca2+ 4.54 mg/dl, and Mg2+ 1.34 mg/dl; hypokalemia was observed. During dissection of the recipient liver, the patient was hemodynamically stable with the exception of occasional premature ventricular complexes (PVCs), and transfusions of red blood cells were performed because of an initial low hemoglobin level (9.1 g/dl) and surgical bleeding. In addition, magnesium sulfate 1 g was mixed with the running fluid and calcium chloride 300 mg was administered repeatedly in order to manage low levels of Mg2+ and Ca2+ reported in electrolyte studies conducted at intervals of one hour.
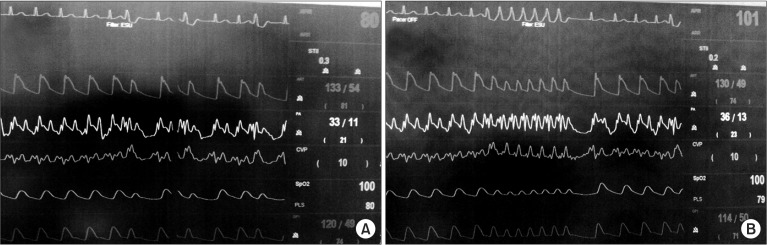
Following removal of the recipient liver, basiliximab 20 mg mixed in normal saline 100 ml was intravenously administered. During partial clamping of the inferior vena cava, ventricular fibrillation with loss of the arterial pressure wave was observed on the ECG. After approximately thirty seconds, normal sinus rhythm returned with unclamping of the inferior vena cava. After a while, clamping of the inferior vena cava was attempted again, but ventricular fibrillation with hemodynamic instability reappeared. The inferior vena cava was thus unclamped immediately, and normal sinus rhythm was then spontaneously restored. Thereafter, ventricular fibrillation and PVCs were observed intermittently regardless of clamping of the inferior vena cava (Fig. 2A), so intravenous lidocaine 50 or 100 mg was repeatedly administered to maintain normal sinus rhythm. According to the cardiologist's recommendation, continuous infusions of lidocaine (2 mg/kg/hr) and isoproterenol (1 µg/min) were initiated under the impression of TdP. The infusion dose of isoproterenol was gradually increased to obtain the desired heart rate of 90-100 bpm. In addition, in order to rapidly cope with ventricular arrhythmia and hemodynamic compromise, external multifunction electrode pads (Philips Medical Systems, Andover, MA, USA) for defibrillation and non-invasive pacing were applied to the chest of the patient before reperfusion. No further episodes of ventricular arrhythmia were observed after these preventive measures. During the reperfusion period, transient hemodynamic instability was adequately managed with intravenous epinephrine 100 µg. After completion of surgery, the patient was transferred to the intensive care unit (ICU) under continuous infusion of lidocaine and isoproterenol. Following admission to the ICU, the patient's initial QT interval was more prolonged (QT/QTc 520/646) compared to baseline. However, the QT interval gradually shortened, and further episodes of ventricular arrhythmia did not occur. Thus, the infusions of lidocaine and isoproterenol were tapered and discontinued on postoperative day (POD) 3 and the patient was transferred to the general ward on POD 5.
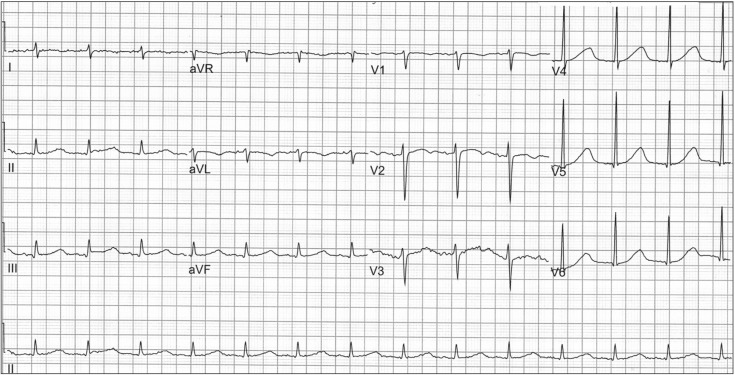
A 54-year-old, 175 cm, 66 kg male patient underwent liver transplantation due to alcoholic liver cirrhosis and hepatic encephalopathy. The patient had a history of heavy alcoholism, pulmonary tuberculosis, and intestinal tuberculosis. Before surgery, he was admitted to the ICU for management of aggravated hepatic encephalopathy. A preoperative ECG revealed normal sinus rhythm, right axis deviation, and prolonged QT interval (QT/QTc 524/612) (Fig. 3), and transthoracic TTE performed one month earlier had shown a left ventricular ejection fraction of 71%, a relaxation abnormality of the left ventricular filling pattern (E/A ratio = 0.84 and E/E' = 9.7), and a right ventricular systolic pressure of 43.7 mmHg. After induction of anesthesia, the patient's hemodynamic variables were as follows: systemic blood pressure 128/55 mmHg, heart rate 82 bpm, central venous pressure 9 mmHg, and pulmonary arterial pressure 29/13 mmHg. The results of the initial electrolyte studies showed Na+ 125.2 mmol/L, K+ 2.8 mmol/L, Cl- 110 mmol/L, Ca2+ 4.24 mg/dl, and Mg2+ 1.37 mg/dl; hyponatremia, hypocalcemia, and hypokalemia were observed. Calcium choride 300 mg was repeatedly administered to correct hypocalcemia, and magnesium sulfate 2 g was mixed with the running fluid. During the dissection phase, two transient episodes of ventricular tachycardia with hemodynamic instability occurred and PVCs were frequently observed (Fig. 2B). In order to maintain normal sinus rhythm, lidocaine 100 mg was intravenously administered twice. Under the impression of TdP, infusion of isoproterenol (1 µg/min) was initiated, and the infusion dose was gradually increased to establish the target heart rate of 90-100 bpm. In addition, potassium replacement was initiated to correct hypokalemia. Following these preventive managements, the patient experienced no further episodes of ventricular arrhythmia during the remainder of the operation, including the reperfusion period. After completion of surgery, the patient was transferred to the ICU under continuous infusion of isoproterenol. Following admission to the ICU, the patient's initial QT interval was somewhat improved (QT/QTc 482/549) compared with the baseline value. The QT interval gradually shortened, and further episodes of ventricular arrhythmia were not observed. Hence, the infusion of isoproterenol was tapered and discontinued on POD 7 and the patient was then transferred to the general ward.
Go to : 
In recent years, the concept of cirrhotic cardiomyopathy as a type of chronic cardiac dysfunction in patients with liver cirrhosis has been confirmed with an increasing amount of evidence, and it has been characterized by blunted ventricular response in stressful situations in spite of normal or augmented systolic function at rest, altered diastolic function, and abnormalities of cardiac electrophysiology including prolonged time interval between electrical and mechanical systole, QT interval prolongation, and blunted tachycardiac response to stress [6].
Prolonged QT interval as the typical electrophysiological change is observed in up to 50% of patients with liver cirrhosis [6]. Although the first report of QT interval prolongation was investigated in patients with alcoholic liver cirrhosis, it is not related to the etiology of liver cirrhosis [7]. A previous report demonstrated that the prolonged QT interval is associated with the severity of liver dysfunction and the circulating level of plasma norepinephrine, which is related to sympathetic activity [8]. As increased sympathetic activity is the primary feature of the hyperdynamic circulatory syndrome in advanced liver disease, this activity has been proposed to be a cause of the prolonged QT interval [7]. As evidence to support this proposal, Zambruni et al. [9] reported that chronic administration of β-blocking agents shortened the QT interval in patients with prolonged QT interval.
It has been suggested that the prolonged QT interval is associated with portal hypertension, as the QT interval is commonly prolonged in both non-cirrhotic and cirrhotic patients with portal hypertension [10]. The pathogenetic mechanism by which portal hypertension may prolong the QT interval has been described as the dumping into the systemic circulation of cardiotoxic substances such as bile salts, endotoxins, cytokines, and insulin produced in the splanchnic area. These cardiotoxic materials may provoke damage of cardiac cell membranes and alteration of ion channels. In addition, the insertion of a transjugular intrahepatic portosystemic shunt (TIPS) has been shown to aggravate the prolonged QT interval because the portosystemic shunt flow enhanced by TIPS increases the amount of toxic substances entering into the systemic circulation [7,10].
It has been well reported that prolonged QT interval can provoke fatal ventricular arrhythmias, which can result in sudden death [1,2]. Day et al. [4] demonstrated that the incidence of sudden death was greatly increased in patients with alcoholic liver disease and prolonged QT interval. As there is the general perception that sudden death is not common in patients with liver cirrhosis, the clinical significance of prolonged QT interval related to liver cirrhosis should be ascertained [7]. However, the incidence of these complications may be underestimated because ECG monitoring is not frequently performed even in cirrhotic patients with severe comorbidities such as bacterial infections and gastrointestinal bleeding [7]. These conditions can lead to additional and abrupt elevations of sympathetic activity, and the use of drugs such as quinolones and vasopressin analogues to treat them can increase the risk of arrhythmias including TdP [7]. Therefore, adequate monitoring and management to prevent the fatal arrhythmias should be considered during anesthesia for cirrhotic patients with QT interval prolongation under these stressful complications.
It has been demonstrated that TdP can occur due to myocardial ischemia, electrolyte imbalance, and drugs that affect QT interval during liver transplantation in patients with prolonged QT interval [5]. In a recent study measuring QT interval during liver transplantation, QT interval gradually increased from the pre-anhepatic phase to the neohepatic phase compared to the baseline value, and prolonged QT interval was maintained without recovery even in the neohepatic phase. In addition, severe prolongation of QT interval (QTc ≥ 500 ms) in the anhepatic phase was observed even in 36% of patients with baseline QTc ≤ 440 ms [5]. In our cases, severe prolonged QT intervals (QTc = 521 and 621 ms) were observed on the preoperative ECGs of both patients, and the patient in the first case had a more prolonged QT interval compared to baseline on the postoperative ECG performed just after ICU admission. Thus, preoperative QT interval prolongation and its further increase during surgery might contribute to the occurrence of ventricular arrhythmia in our cases.
In order to prevent severe ventricular arrhythmia in patients with prolonged QT interval, optimization of electrolyte balance and hemodynamic status is preferentially required during liver transplantation, and drugs that can prolong QT interval should be avoided as much as possible [2,5]. In addition, acceleration of the basal heart rate is effective to prevent the occurrence of TdP. Isoproterenol at a dose of 1-10 µg/min can be applied to increase the heart rate to the desired 90-100 bpm [11]. Cardiac pacing can be also considered as an effective tool to accelerate the heart rate [11,12].
It is concluded that the fatal ventricular arrhythmias related to prolonged QT interval can occur during liver transplantation. Thus, these cases emphasize that preoperative recognition of QT interval prolongation and adequate monitoring and management for the prevention of fatal arrhythmias are important in patients undergoing liver transplantation.
Go to : 
References
1. Booker PD, Whyte SD, Ladusans EJ. Long QT syndrome and anaesthesia. Br J Anaesth. 2003; 90:349–366. PMID: 12594150.

3. Lee JY, Lee JH, An EH, Song JG, Park PH. Postanesthetic torsade de pointes in a patient with unrecognized long QT syndrome: A case report. Korean J Anesthesiol. 2011; 60:294–297. PMID: 21602982.

4. Day CP, James OF, Butler TJ, Campbell RW. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet. 1993; 341:1423–1428. PMID: 8099138.
5. Shin WJ, Kim YK, Song JG, Kim SH, Choi SS, Song JH, et al. Alterations in QT interval in patients undergoing living donor liver transplantation. Transplant Proc. 2011; 43:170–173. PMID: 21335179.

6. Moller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010; 53:179–190. PMID: 20462649.
7. Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat. Expert Rev Gastroenterol Hepatol. 2012; 6:57–66. PMID: 22149582.

8. Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998; 27:28–34. PMID: 9425913.

9. Zambruni A, Trevisani F, Di Micoli A, Savelli F, Berzigotti A, Bracci E, et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008; 48:415–421. PMID: 18194821.
10. Trevisani F, Merli M, Savelli F, Valeriano V, Zambruni A, Riggio O, et al. QT interval in patients with non-cirrhotic portal hypertension and in cirrhotic patients treated with transjugular intrahepatic porto-systemic shunt. J Hepatol. 2003; 38:461–467. PMID: 12663238.

11. Khan IA, Gowda RM. Novel therapeutics for treatment of long-QT syndrome and torsade de pointes. Int J Cardiol. 2004; 95:1–6. PMID: 15159030.

12. Roden DM. A practical approach to torsade de pointes. Clin Cardiol. 1997; 20:285–290. PMID: 9068917.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download