Abstract
We report a case of increased values of entropy parameters Response Entropy (RE) and State Entropy (SE) during intravenous general anesthesia in a sleepwalking patient. An ASA class II, 64-year-old woman with stress incontinence underwent mid-urethral sling surgery. Prior to surgery, the patient had been administered paroxetine, valproic acid and clonazepam for the treatment of sleepwalking disorder. After 10 min of target-controlled infusion of propofol and remifentanil, entropy values increased up to 94 (RE) and 88 (SE) for 10 min. The target effect-site concentrations of anesthetics increased from 4 to 7 µg/ml propofol and 4 ng/ml remifentanil, at which point values fell back to adequate anesthesia levels. Episodes of recall or of explicit memories did not occur during the anesthesia. In conclusion, sleepwalking patients with long-term use medications may need increment of anesthetic dose caused by the anesthetic drug metabolism activation or impairment or immaturity of inhibitory circuits in brain.
Go to : 
Sleepwalking, also known as somnambulism, is a series of complex motor behaviors that result in walking or exerting large movements during deep sleep. This condition is thought to be caused by various factors including drugs [1,2], stress, psychic conditions, sleep deprivation and genetic susceptibility. Sleepwalking occurs in 2-14% of children. Onset occurs commonly before 10 years of age and it is usually outgrown. However, up to 25% of patients continue sleepwalking into adulthood. The prevalence of sleepwalking in adults is 1.6-2.4% [3]. Occasional, benign episodes of sleepwalking require no active treatment. However, patients who experience recurrent sleepwalking with a risk of injury to self or others require immediate pharmacological treatment. Commonly recommended medications are benzodiazepines (particularly clonazepam), tricyclic antidepressants, and selective serotonin reuptake inhibitors.
During general anesthesia, as the brain becomes increasingly saturated with anesthetics, both electroencephalograph (EEG) and frontal electromyograph (fEMG), which measure brain and facial muscle activities, respectively, change from irregular to more regular patterns. The entropy module is a computed device that monitors the depth of anesthesia using data acquisition of EEG and fEMG signals, and provides measurements of the irregularity of EEG and fEMG using an entropy algorithm [4]. The entropy module provides two different entropy parameters, the State Entropy (SE) and the Response Entropy (RE). The value of SE is calculated from the measurement of the entropy of the EEG, which is related to cortical activity; SE has a frequency rate of 0.8-32 Hz and displays a range of 0-91. The value of RE is calculated from the measurement of the entropy of the EEG and the fEMG and has a frequency rate of 0.8-47 Hz. Furthermore, the entropy module has recently been used to evaluate the effects of anesthetic agents during general anesthesia.
In this report, we present a case of increasing EEG entropy during intravenous general anesthesia in a sleepwalking patient.
We present the case of a 64-year-old female patient (American Society of Anesthesiologists physical status class II) with stress incontinence who underwent a mid-urethral sling operation. The patient was diagnosed with sleepwalking 2 years prior to the operation and was prescribed clonazepam (0.5 mg/day), valproic acid (150 mg/day) and paroxetine (20 mg/day). The patient was taking these medications for the 2 year period prior to surgery. The patient's psychiatrist recommended stopping the prescribed medication in the morning of the operation day and restarting after surgery. Routine preoperative investigations were within normal limits (146 cm, 52.1 kg; hemoglobin levels [Hb]:12.3 g/dl).
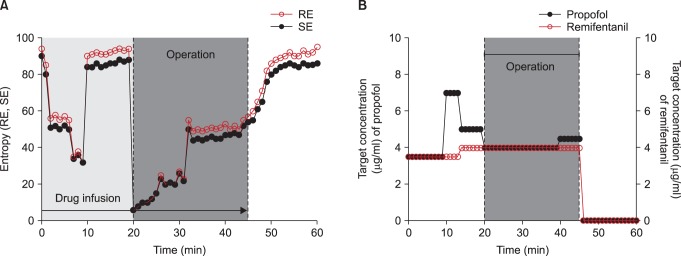
EEG, electrocardiography, end-tidal CO2 partial pressure, pulse oximetry, and noninvasive blood pressure were monitored throughout surgery. Entropy of EEG was determined using an entropy module (M-Entropy™; Datex-Ohmeda, Helsinki, Finland). At the beginning of surgery, the values of RE and SE were 94 and 90, respectively.
Intravenous anesthesia was provided using target-controlled infusion (TCI) of propofol and remifentanil. The infusions of propofol and remifentanil were prepared using fresofol 2% injection, 50 ml vial (Fresenius Kabi, Austria) and Ultiva™injection, 1 mg vial (GlaxoSmithKline, Belgium), respectively. Remifentanil 1 mg was diluted into 50 ml of normal saline (20 µg/ml solution). Both infusions were prepared in 50 ml syringes. A commercial TCI pump (Orchestra® Base Primea, Fresenius Vial, France) was used for the target effect-site TCI of propofol and remifentanil. The pump used the models of Marsh and colleagues for propofol [5] and Minto and colleagues for remifentanil [6]. The TCI device was adjusted to deliver target effect-site concentrations of 3.5-7 µg/ml propofol and 3.5-4 ng/ml remifentanil depending on the condition of the patient. Initial target effect-site concentrations of propofol and remifentanil were 3.5 µg/ml and 3.5 ng/ml, respectively.
After induction of anesthesia, RE and SE were maintained within the range of the anesthetic (Fig. 1). The laryngeal mask airway was introduced 3 min after the start of anesthetic drug infusion. Mechanical ventilation was maintained using oxygen with air (FiO2 0.5) at a constant tidal volume (10 ml/kg) and frequency (10 breaths/min). There was a sudden increase of RE from 32 up to 94 and SE from 32 up to 88, 10 min after the start of anesthetic drug infusion. Target effect-site propofol concentration was increased from 3.5 to 7 µg/ml for 5 min, and then decreased to 5 µg/ml. Target effect-site remifentanil concentration was increased from 3.5 to 4 ng/ml. We suspected that the sudden increase in entropy may have been due to blockage of the intravenous line. Drug infusion line was connected using 3 way stopcock directly to the angio-cath; However, we failed to find a blockade in the intravenous line and concluded that propofol and remifentanil were being infused in a satisfactory manner. In addition, there were no nociceptive stimuli at this time because surgeons were in the process of preparing the surgical procedure. There were no signs of patient movement or arousal. Both RE and SE were maintained at approximately 90 for 10 min. After this time, RE and SE both suddenly decreased from 94 and 88 to below 10, and the operation started.
During the operation, target effect-site concentrations of propofol and remifentanil were maintained at 4 µg/ml and 4 ng/ml, respectively. Entropy values of RE and SE gradually increased during the operation to reach levels of consciousness (84 and 80) at the end of surgery. Propofol and remifentanil infusion was discontinued 5 min after the end of operation.
The total dose of propofol and remifentanil delivered was 600 mg and 400 µg, respectively, during the 50 min of operation time. Heart rate and mean blood pressure stayed within 20% of baseline values throughout the surgery (Fig. 1). The patient was transferred to the post-anesthesia care unit 10 min after cessation of propofol and remifentanil infusion, and the last values of RE and SE were 95 and 86.
The patient was questioned about awareness during the operation at three times points (immediately after surgery, prior to discharge at 72 h post-surgery, and 1 month post-surgery) using the modified Brice questionnaire (Table 1) [7,8]. Answers were consistent across all three time points. Therefore, we conclude that there was no clear recall or explicit memories during general anesthesia.
Go to : 
Here, we report of the use of an entropy module to measure the sedation in an adult sleepwalking patient with drug medication during intravenous anesthesia. The SE and RE values provided by the entropy module can provide reliable information about the level of sedation during surgery. Several studies suggest that SE and RE are useful parameters for determining the anesthetic effects of propofol, of the combination of propofol and remifentanil, and of sevoflurane.
Anesthetic requirement varies with a patient's age, clinical conditions, disease, and administration of other drugs. In this case, the patient was diagnosed with sleepwalking 2 years prior to the operation and has been administered benzodiazepines, valproic acid and selective serotonin reuptake inhibitors. Therefore, the patient's disease or possible drug interactions might have been responsible for an increase in anesthetic requirement and caused the unexpected increase in EEG entropy that occurred 10 min into surgery.
Psychiatric drugs usually given in combination with other, non-psychiatric drugs, or with each other, can often have an effect on peripheral or central neurotransmitter systems and ionic balances. Benzodiazepine-like clonazepam is known to increase blood concentrations of propofol by 25% through a decrease in the metabolic, rapid, and slow distribution clearance pathways of propofol; a decrease in mean arterial blood pressure would cause pharmacokinetic alterations of propofol and increase the blood propofol concentrations even further. Valproic acid may have significantly reduced the required dose of propofol due to its inhibitory action on the metabolic enzymes responsible for the metabolism of propofol [9]. Finally, propofol is known to inhibit norepinephrine and serotonin transporter protein functions in a drug-dependent manner. Therefore, selective serotonin reuptake inhibitors may be expected to alter the response of norepinephrine and serotonin transporter proteins to propofol. The psychiatric medications taken by our patient would be expected to decrease the anesthetic requirement.
Furthermore, our patient has been exposed to psychiatric medications for a period of 2 years. Propofol is metabolized primarily by glucuronidation through UDP-glucuronosyl-transferase 1A9. The cytochrome (CYP) enzymes CYP2B6, CYP3A4 and CYP1A2 play an important role in the liver metabolism of propofol, which is performed by oxidation of propofol via ring hydroxylation [10,11]. Under long-term use of psychiatric medications, the metabolism of the anesthetic drug can be changed such as transcriptional activation leading to increased synthesis of CYP 450 enzyme proteins. As a result, blood levels of the medication decrease, generally over the first 3 months of exposure to the medication [12]. This case also can be explained in the same way.
The measured EEG signal is primarily generated by cortical pyramidal cells and is the result of complex cortico-cortical and cortico-subcortical interactions. As such, diseases involving these structures or their connections may be expected to influence the EEG signal. For example, the EEG of patients with Alzheimer's disease or multi-infarct dementia has a lower bispectral index than the EEG of normal 'awake' adults, which correlates with the level of cognitive impairment in these patients [13]. Sleepwalking disorder may involve similar alterations within brain structures or connections. In support of this hypothesis, single photon emission computed tomography (CT scans) performed in patients during sleepwalking episodes reveal a persistent deactivation of thalamocortical arousal system and the thalamocingulate pathway as a dissociated state with concomitant motor arousal and sleep of mind. Furthermore, Oliviero and colleagues [14] suggested that functional abnormality of arousal circuits that widely project to the cortex could lead to changes in cortical excitability in adult sleepwalkers, even during wakefulness. The authors reported that adult sleepwalkers had reduced levels of short interval intracortical inhibition, reduced cortical silent period duration, and reduced short latency afferent inhibition during wakefulness. Their results suggested that sleepwalkers may have an impairment of inhibitory circuits that represents the immaturity of some neural circuits, synapses or receptors in the brain [14]. Therefore, the aforementioned changes may lead to drug resistance and EEG changes. This possibility is supported by the finding that patients who present with autism spectrum disorder as a result of deficient neural development have greater propofol requirements for anesthesia during ordinary dental treatment compared with intellectually impaired patients [15].
Finally, monitoring the sedation does not always coincide with a clinically-monitored level of consciousness. Cardiac pacing devices, electromagnetic systems, endoscopic shaver devices, forced-air warming devices and wire-reinforced EMG tubes can all have effects on the measured entropy value. However, in this case, the use of machinery or even the nociceptive stimuli that can affect the EEG or the entropy values may not be given during preparation of the surgical procedure.
In conclusion, sleepwalking patients with long-term use of psychiatric medications may need the increment of anesthetic requirement. It may be caused by the activation of the anesthetic drug metabolism leading to increased synthesis of CYP 450 enzyme proteins or an impairment or immaturity of inhibitory circuits due to somnambulism. Therefore, anesthesiologists may wish to consider a possible change in anesthetic requirement depending on the type of disease and prescribed medications. Furthermore, anesthesia depth monitoring through the use of devices such as an entropy module is recommended for the safety of patients.
Go to : 
References
1. Charney DS, Kales A, Soldatos CR, Nelson JC. Somnambulistic-like episodes secondary to combined lithium-neuroleptic treatment. Br J Psychiatry. 1979; 135:418–424. PMID: 44211.

2. Harazin J, Berigan TR. Zolpidem tartrate and somnambulism. Mil Med. 1999; 164:669–670. PMID: 10495642.

3. Hublin C, Kaprio J, Partinen M, Heikkilä K, Koskenvuo M. Prevalence and genetics of sleepwalking: a population-based twin study. Neurology. 1997; 48:177–181. PMID: 9008515.

4. Kwon MY, Lee SY, Kim TY, Kim DK, Lee KM, Woo NS, et al. Spectral entropy for assessing the depth of propofol sedation. Korean J Anesthesiol. 2012; 62:234–239. PMID: 22474549.

5. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991; 67:41–48. PMID: 1859758.

6. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997; 86:10–23. PMID: 9009935.
7. Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. Br J Anaesth. 1970; 42:535–542. PMID: 5423844.

8. Pollard RJ, Coyle JP, Gilbert RL, Beck JE. Intraoperative awareness in a regional medical system: a review of 3 years' data. Anesthesiology. 2007; 106:269–274. PMID: 17264720.
9. Wen X, Wang JS, Kivisto KT, Neuvonen PJ, Backman JT. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: preferential inhibition of cytochrome P450 2C9 (CYP2C9). Br J Clin Pharmacol. 2001; 52:547–553. PMID: 11736863.

10. Murayama N, Minoshima M, Shimizu M, Guengerich FP, Yamazaki H. Involvement of human cytochrome P450 2B6 in the omega- and 4-hydroxylation of the anesthetic agent propofol. Xenobiotica. 2007; 37:717–724. PMID: 17620218.
11. Yamazaki H, Shimizu M, Nagashima T, Minoshima M, Murayama N. Rat cytochrome P450 2C11 in liver microsomes involved in oxidation of anesthetic agent propofol and deactivated by prior treatment with propofol. Drug Metab Dispos. 2006; 34:1803–1805. PMID: 16896062.

12. Tokola RA, Neuvonen PJ. Pharmacokinetics of antiepileptic drugs. Acta Neurol Scand Suppl. 1983; 97:17–27. PMID: 6143468.

13. Gutman DA, Owens MJ. Serotonin and norepinephrine transporter binding profile of SSRIs. Essent Psychopharmacol. 2006; 7:35–41. PMID: 16989291.
14. Oliviero A, Della Marca G, Tonali PA, Pilato F, Saturno E, Dileone M, et al. Functional involvement of cerebral cortex in adult sleepwalking. J Neurol. 2007; 254:1066–1072. PMID: 17351721.

15. Asahi Y, Kubota K, Omichi S. Dose requirements for propofol anaesthesia for dental treatment for autistic patients compared with intellectually impaired patients. Anaesth Intensive Care. 2009; 37:70–73. PMID: 19157349.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download