This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The aim of this randomized, double-blind, placebo-controlled study was to evaluate dose effects of ephedrine pretreatment on the onset time and intubating conditions after cisatracurium administration.
Methods
A total of 140 adult patients were randomized into 4 groups to receive either 30 µg/kg ephedrine (Group 30, n = 35), 70 µg/kg ephedrine (Group 70, n = 35), 110 µg/kg ephedrine (Group 110, n = 35), 3 ml normal saline (Group C, n = 35) as pretreatment given 30 s before anesthetic induction. Neuromuscular block was achieved with 0.15 mg/kg cisatracurium, evaluated accelomyographically with train-of-four stimulation. An anesthesiologist blinded to patient grouping assessed the intubating conditions 1.5 min after cisatracurium administration.
Results
An onset time of 70 s was obtained in the ephedrine groups (Group 30: 155.4 ± 44.7 s, Group 70: 152.6 ± 40.3 s, Group 110: 151.2 ± 51.6 s) compared to Group C (224.6 ± 56.9 s) after 0.15 mg/kg of cisatracurium (P < 0.001). Ephedrine doses of either 70 or 110 µg/kg for pretreatment significantly improved intubating conditions (P < 0.05). Systolic and diastolic blood pressure and heart rate at 1 min after tracheal intubation were significantly increased than other times in all groups (P < 0.001), with no differences among the groups. However, 5 patients in Group 110 experienced marked hypertension (systolic/diastolic blood pressure: > 200/100 mmHg) 1 min after tracheal intubation with no patients in other groups.
Conclusions
We conclude that pre-treatment with ephedrine 70 µg/kg improved intubating conditions 1.5 min after cisatracurium administration and facilitated the onset of neuromuscular block (70 s) without adverse hemodynamic effects.
Go to :

Keywords: Cisatracurium, Ephedrine, Hemodynamics, Intubation
Introduction
Cisatracurium, a benzyl isoquinoline compound of the intermediate-acting neuromuscular blockade (NMB) class, has high potency, causes a minor release of histamine, less laudanosine production, undergoes organ-independent Hofmann elimination, and has a slower onset time [
1,
2]. In adults, cisatracurium given at a dose of 0.15 mg/kg (3 × ED
95) has been found to have an onset time of 220 s, which is much slower than succinylcholine or rocuronium [
3]. However, several trials with ketamine, magnesium sulfate, ephedrine, and priming dose of cisatracurium, have reported to techniques reduce the onset time of cisatracurium and improve intubating conditions [
4,
5,
6,
7,
8].
The onset time of cisatracurium was reduced by 40% when pre-treatment with ephedrine 70 µg/kg was administered [
7]. A low-dose of ephedrine (70 µg/kg) plus a priming dose with cisatracurium before an intubating dose improved clinical intubating conditions by 36% at 60 s after the intubating dose of cisatracurium [
6]. However, the hemodynamic response to laryngoscopy and endotracheal intubation was significantly increased during the induction [
9]. Anesthesiologists should be aware of the potential adverse hemodynamic consequences of ephedrine pretreatment prior to tracheal intubation. Therefore, an optimal dose of ephedrine should be determined to minimize adverse hemodynamic responses during the induction of anesthesia. We chose a dose of ephedrine as 30, 70 and 110 µg/kg, to know the effects of small and large than previous reported dose (70 µg/kg) [
6,
7].
In this study, we compared the influence of saline and ephedrine 30, 70, or 110 µg/kg given before induction on (1) intubating conditions 1.5 min after cisatracurium 0.15 mg/kg and (2) hemodynamic changes (heart rate and systolic and diastolic blood pressure) during induction time.
Go to :

Materials and Methods
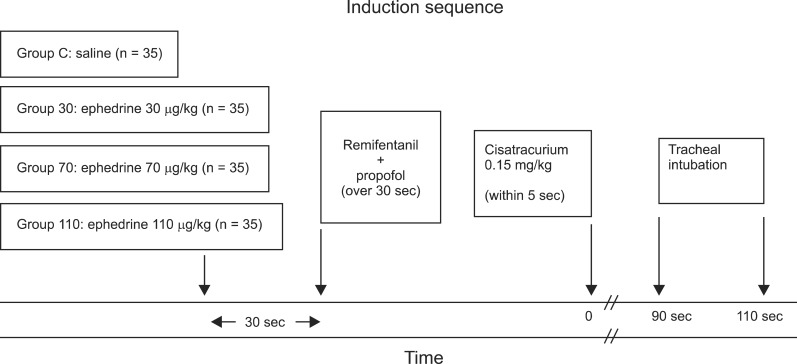
Before this study was performed, approval (IRB File No.: 2012-06-026) was obtained from the Hospital Ethics Committee and written informed consent was obtained from all patients. Adult patients (n = 140) aged 20 to 64 years with an American Society of Anesthesiologists physical status I or II who underwent elective surgery under general anesthesia with tracheal intubation in the supine position were enrolled in this study. Using a computer-generated program, 35 patients were allocated randomly to one of four study groups: pretreatment with ephedrine 30 µg/kg (Group 30), ephedrine 70 µg/kg (Group 70), ephedrine 110 µg/kg (Group 110), or 3 ml NaCl 0.9% (Group C) given 30 s before anesthesia induction (
Fig. 1). Patients were excluded for the following reasons: those expected to have a difficult airway, neuromuscular, hepatic, or renal diseases, ischemic heart disease or a history of myocardial infarction within the last year, pregnancy and those with a body weight 20% greater than their ideal body weight.
 | Fig. 1
|
No premedication was given. On arrival in the operating room, automatic monitoring with noninvasive arterial blood pressure, oxygen saturation and electrocardiography (ECG) was begun. Bispectral index (BIS) was monitored using a BIS XP monitor (Model A 2000, Aspect Medical Systems, Natick, MA, USA) and was measured every 10 s during anesthesia.
Anesthesia was induced with remifentanil 0.5 µg/kg/min injected intravenously over 2 min followed by midazolam 3 mg and propofol 1.5 mg/kg injected intravenously over 30 s by an anesthesiologist blinded to group assignment. As soon as the patient lost consciousness, ventilation via facemask with 50% oxygen-enriched air was manually administered to keep the end-tidal carbon dioxide tension within the range of 30 to 35 mmHg until tracheal intubation. Anesthesia was maintained with propofol (3 mg/kg/h) and remifentanil (0.1-0.3 µg/kg/min) with the goal of maintaining the BIS below 50.
Neuromuscular function was assessed using acceleromyography of the adductor pollicis muscle (TOF-Watch SX®, Organon Ltd., Dublin, Ireland). Two electrodes (Cleartrode™, Ref 1720-003, ConMed®, Utica, NY, USA) were placed over the ulnar nerve of the hands and an accelerometer was fixed to the volar aspect of the thumb. Neuromuscular monitoring began immediately when the patient lost consciousness. A 5-s 50-Hertz (Hz) supramaximal tetanic stimulus was administered for calibration of acceleromyography [
10]. Calibration was performed using the CAL2 mode of the TOF-Watch SX®, which calibrates automatically by searching for the supramaximal level. Train-of-four (TOF) stimulations (0.2 ms in duration at 2 Hz with supramaximal current) were applied every 15 s during anesthesia. All neuromuscular data was collected on a laptop computer.
Cisatracurium (0.15 mg/kg) was administered rapidly within 5 s after stabilization of control responses. Tracheal intubation was performed 1.5 min after cisatracurium within 20 s by an anesthesiologist blinded to the protocol and with at least 3 years of experience. The same physician also assessed the intubating conditions. Intubating conditions were graded according to the criteria of Fuchs-Buder et al. [
11]: easy of laryngoscopy (0 = difficult, 1 = fair, 2 = easy), position of vocal cords (0 = closed, 1 = intermediate/moving, 2 = adducted), reaction to intubation (diaphragmatic movement/coughing) (0 = vigorous/sustained, 1 = slight, 2 = none). A score of 5 or 6 was considered excellent, 3 or 4 good, and 0 to 2 poor. The point of disappearance of the first twitch of TOF stimulation was recorded as the onset time of cisatracurium at the adductor pollicis. A diagram of the induction is shown in
Fig. 1. Skin temperature on the hand was kept above 32℃ by wrapping the arm in cotton wool and core temperature was kept above 35℃ with a corrugated heating circuit and air warming.
Systolic and diastolic blood pressure, heart rate and BIS were measured and recorded at the following times: preinduction, immediately prior to intubation, and 1 min and 3 min after intubation. A 20% change in hemodynamic variables from baseline values was regarded as an adverse effect. The presence of arrhythmias on the ECG monitor was recorded. All patients were interviewed by a blinded investigator 24 h after surgery and assessed for awareness during anesthetic induction.
Statistical analyses were performed using SPSS statistical software (Windows ver. 19.0, SPSS Inc, Chicago, IL, USA). In this study, sample size calculations were taken from a previous study [
7]. On the basis of a relevant 20% change (SD 24% change) in the onset time (167 ± 64.8 s) of cisatracurium, we calculated that 32 patients would be needed to test the null hypothesis at a significance of 0.05 and a power of 0.80. We enrolled 35 patients to account for a 10% dropout rate. One-way analysis of variance (ANOVA) was performed for continuous variables (i.e., age, weight, height, anesthetic time and BIS). To study the effects of time and group allocation on each of the variables, repeated-measure ANOVA was used to compare the hemodynamic parameters between preinduction values and time (three levels). When the results were significant, the Student-Newman-Keuls post-test was used to localize significant differences. One-way ANOVA was used between groups for onset time and multiple comparisons were made with the Dunn's method if a significant difference was found. The Kruskal-Wallis ANOVA with Tukey's test for post hoc analysis was used to determine the significance of differences with respect to the intubation conditions. The data were expressed as the mean ± SD (number, %). P values < 0.05 were considered statistically significant.
Go to :

Results
Among the 140 patients recruited for this study, none were excluded. No significant differences in age, gender, weight, height, anesthetic time or BIS were observed between groups (
Table 1). The onset time of cisatracurium 0.15 mg/kg was shorter after pre-treatment with ephedrine (Group 30: 155.4 ± 44.7 s, Group 70: 152.6 ± 40.3 s, Group 110: 151.2 ± 51.6 s) compared to placebo (Group C: 224.6 ± 56.9 s) (P < 0.001) (
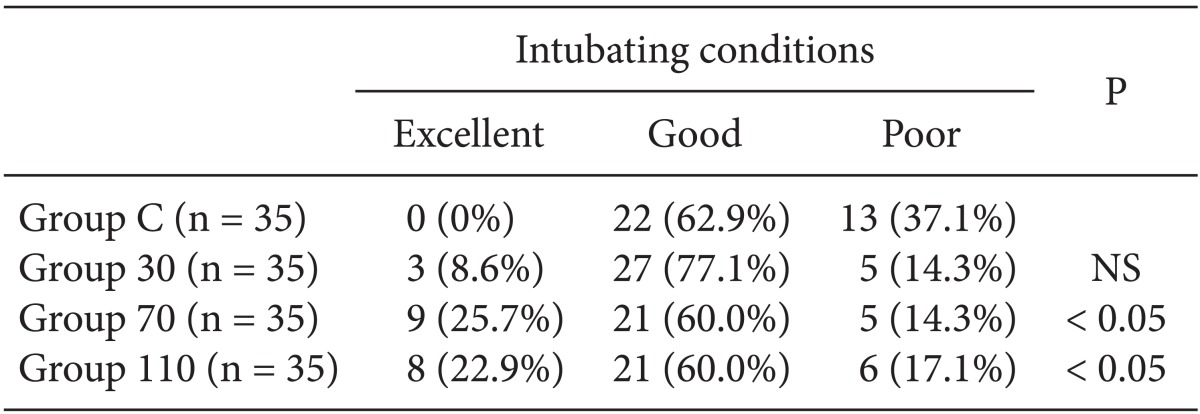
Fig. 2). Overall excellent and good intubating conditions were significantly increased after larger ephedrine doses (Group 70 and Group 110; 25.7 and 60, 22.9 and 60%, respectively) than placebo (0 and 62.9%) (P < 0.05), but there were no differences between doses of ephedrine (
Table 2). Systolic and diastolic blood pressure and heart rate at 1 min after tracheal intubation were significantly increased than preinduction and before intubation in all groups (P < 0.001) (
Figs. 3 and
4), but there were no differences among the groups. Endotracheal intubation caused significant increases in systolic and diastolic blood pressure (systolic/diastolic blood pressure: > 200/100 mmHg) at 1 min in 5 patients (14%) of Group 110 with no patients in other groups (
Fig. 3). No arrhythmias occurred during the study period.
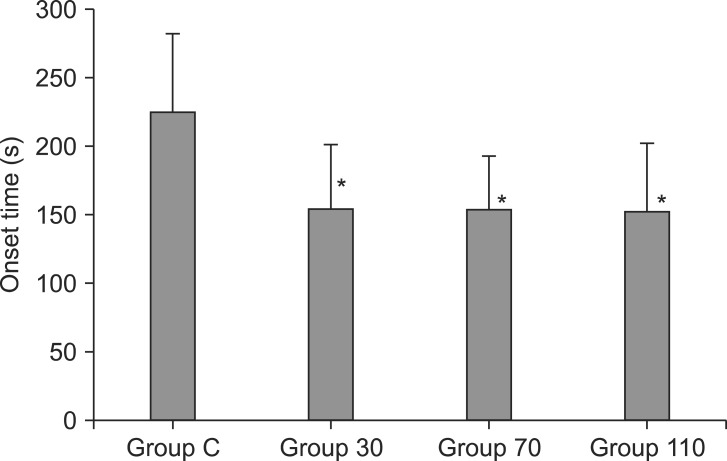 | Fig. 2Onset time of block after cisatracurium 0.15 mg/kg in each group (n = 35). Values are mean ± SD. Group C = saline; Group 30 = ephedrine 30 µg/kg; Group 70 = ephedrine 70 µg/kg; Group 110 = ephedrine 110 µg/kg. *P < 0.001 versus Group C. 
|
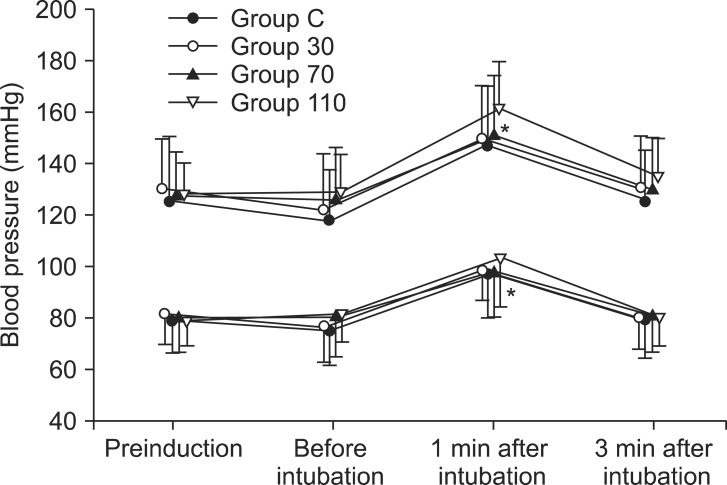 | Fig. 3Changes in systolic and diastolic blood pressure in each group (n = 35) during induction. Values are mean ± SD. Group C = saline; Group 30 = ephedrine 30 µg/kg; Group 70 = ephedrine 70 µg/kg; Group 110 = ephedrine 110 µg/kg. *P < 0.001 versus preinduction and before intubation levels. 
|
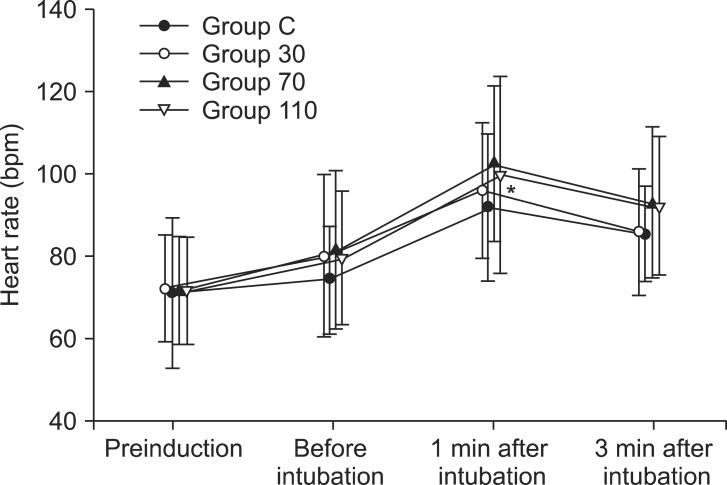 | Fig. 4Changes in heart rate in each group (n = 35) during induction. Values are mean ± SD. Group C = saline; Group 30 = ephedrine 30 µg/kg; Group 70 = ephedrine 70 µg/kg; Group 110 = ephedrine 110 µg/kg. *P < 0.001 versus preinduction and before intubation levels. 
|
Table 1
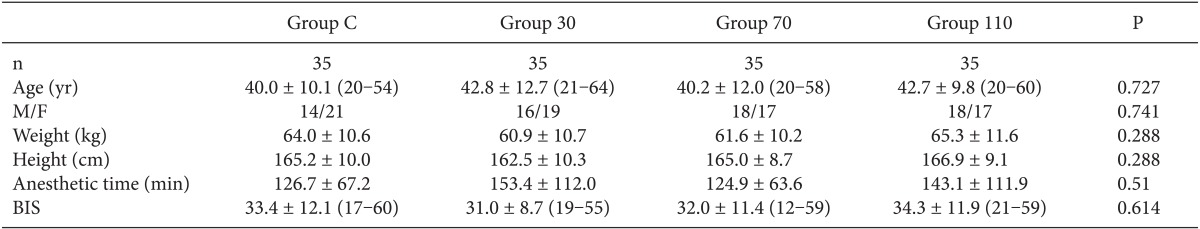

Table 2

Go to :

Discussion
The main finding of this study was that pre-treatment with ephedrine 70 and 110 µg/kg reduced the onset time (70 s) of cisatracurium and improved intubating conditions 1.5 min after cisatracurium. However, ephedrine 110 µg/kg was associated with marked hypertension (systolic/diastolic blood pressure: > 200/100 mmHg) after tracheal intubation.
In the previous study, cisatracurium had a decreased onset time with increased doses (0.2 mg/kg) up to 35% [
3]. Although this increasing dose could reduce the onset time of cisatracurium, adverse effects may occur, such as the development of muscle weakness or increased action duration of neuromuscular blockade [
3]. The priming technique is an alternative to shorten the onset time of NMB. However, fractional cisatracurium doses have not been shown to shorten the NMB onset time as compared to bolus injections [
8,
12]. The combination of low-dose ephedrine (70 µg/kg) and a low priming dose (0.005 mg/kg) of cisatracurium improved clinical intubating conditions at 60 s after the intubating dose of cisatracurium compared to priming without ephedrine or ephedrine without priming [
6]. However, awake patients may suffer from serious adverse events such as blurred vision, diplopia, heavy eyelids, muscle weakness, difficulty swallowing, and voice disorders during the relatively long priming interval [
13]. Additionally, this previous study did not simultaneously evaluate the degree of NMB after atracurium administration. In our study, an onset time of 70 s was obtained after pre-treatment with ephedrine compared to the placebo after 0.15 mg/kg of cisatracurium. Although onset time at the larynx was different than that at the adductor pollicis [
14], we suspect that pre-treatment with ephedrine may also reduce the onset time at the larynx after cisatracurium, which may be a contributing factor to improve intubating conditions.
Occasionally, the hemodynamic responses to propofol during the induction sequence were obtunded by pre-treatment with ephedrine [
15]. However, in most patients, tachycardia and hypertension were exacerbated by tracheal intubation when the pretreatment of ephedrine was added. The use of the ephedrine/propofol mixtures for elderly patients was not recommended due to the risk of tachycardia-inducing myocardial ischemia [
16]. In our study, evidence of the association between ephedrine and marked hypertension was seen 1 min after intubation in 5 patients after pretreatment with ephedrine 110 µg/kg. Pretreatment with ephedrine 30 µg/kg had stable hemodynamic values but no improvement in intubating conditions. However, ephedrine 70 µg/kg was the optimal dose and was found to minimize adverse effects, reduce onset time of cisatracurium and improve intubating conditions.
The rapidity of the onset of NMB at the adductor pollicis muscle was clearly related to cardiac output (CO) [
17]. This study is limited in that we made no measurements of cardiac output and thus we cannot confirm the time to peak effect of ephedrine as related to blood flow. In our previous study, CO and systemic vascular resistance after preinjection of ephedrine were greater until 1 min after tracheal intubation compared with placebo [
18]. We suspect that increased cardiac output due to ephedrine administration may contribute to the rapid onset of cisatracurium.
Although severe adverse effects after the pretreatment with ephedrine were not found in relatively healthy patients from this study, we should consider that a larger dose of ephedrine is not indicated in patients with myocardial ischemia and hypertension due to the possibility of serious adverse effects.
In conclusion, pretreatment with ephedrine 70 µg/kg improved intubating conditions 1.5 min after cisatracurium administration and facilitated the onset of cisatracurium (70 s) without adverse hemodynamic effects.
Go to :









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download