Abstract
Postoperative visual loss (POVL) after non-ophthalmic surgery is rare, with a reported incidence ranging from 0.013 to 0.2%. Most perioperative visual loss is associated with spine operations and cardiac bypass procedures. The most common cause of POVL is ischemic optic neuropathy. However, there are no previous reports of postoperative visual loss after laparoscopic appendectomy. A 43-year-old female with no underlying disease underwent laparoscopic appendectomy; the operation was completed in one hour and her blood pressure was stable during the perioperative period. In the post-anesthetic care unit, the patient complained of nausea and headache, but she did not complain of any unusual visual symptoms. Approximately one hour after arriving at the ward, the patient complained of visual disturbance. Neurologic examination revealed left homonymous hemianopsia, and subarachnoid hemorrhage and intracerebral hemorrhage were found in the occipital area on brain MRI.
Go to : 
In non-ophthalmic surgery, POVL describes either unilateral or bilateral visual loss that occurs after general surgery not including cardiac, lower spine, vascular or reconstructive surgery. In non-ophthalmic surgery POVL is rare, but can develop as an unexpected and severe complication. It is associated with cardiac, spine, and head and neck surgery [1]. POVL's incidence is 0.013% in non-ophthalmic surgery, 0.11% in heart surgery, and 0.2% in spine surgery [2,3,4]. The most common cause of POVL is optic ischemia, and related factors include prone position, duration of surgery, hypotension, massive bleeding and increased venous pressure. However, the exact cause of POVL is still unknown [4]. Moreover, Chaudhry et al. reported that POVL caused by a short period of abdominal surgery such as cholecystectomy or appendectomy is rare [5]. Here, we report POVL associated with intraabdominal hemorrhage after laparoscopic appendectomy.
A 43-year-old female patient, weighting 53 kg visited our hospital due to nausea and abdominal pain. The patient had no underlying disease, did not take any medications, and demonstrated no special features on electrocardiography, biochemical examination of blood, or chest X-ray. Vital signs before anesthesia were blood pressure 140/70 mmHg, heart rate 80 beats/min, body temperature 37℃, respiratory rate 16 /min and oxygen saturation 100%. Appendicitis was observed on abdominal CT, therefore the decision was made to proceed with laparoscopic appendectomy.
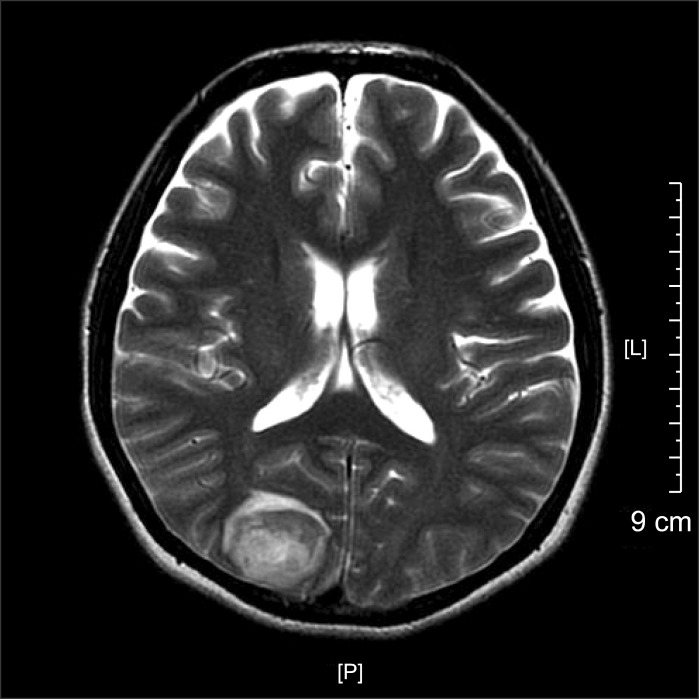
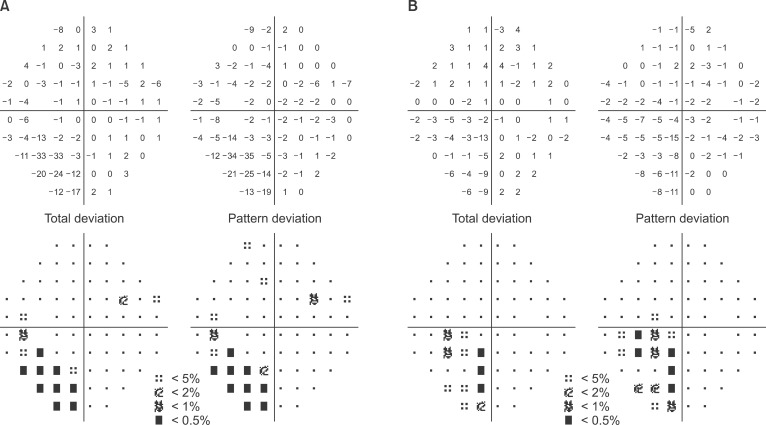
Anesthesia was induced with a bolus injection of lidocaine (1 mg/kg), propofol (1.5 mg/kg), and rocuronium (0.6 mg/kg) in combination with remifentanil infusion at 0.5 µg/kg/min. Anesthesia was maintained with desflurane, remifentanil, and air/oxygen. The patient was placed in Trendelenburg position for the operation. Vital signs after induction were blood pressure 150/80 mmHg, heart rate 100 beats/min and oxygen saturation 100%. Intraoperative vital signs were stable. The patient was transferred to PACU after a one-hour operation. Vital signs in the PACU were blood pressure 140/70 mmHg and heart rate 80 beats/min, but the patient complained of nausea and headache. Her symptoms were relieved after injection of antiemetics, so the patient was transferred to the general ward. Approximately one hour after arriving on the ward, the patient complained of visual disturbance (white vision). Neurologic examination revealed left homonymous hemianopsia, and right temporo-occipital intracerebral hemorrhage, left posterior temporal intracerebral hemorrhage, right cerebellar, and bilateral frontotemporoparietal subarachnoid hemorrhage were demonstrated on brain MRI and CT (Fig. 1). These radiologic findings are suggestive of hypertensive encephalopathy or vasculitis. The next day, the patient's right-side vision was recovered, but left-side vision was glimmering. In obtaining a postoperative history, we found that the patient had previously complained of headache in the left cerebral hemisphere, and that the patient had experienced nausea for one month. The headache was aggravated after travelling in an airplane two weeks prior. The patient showed no pertinent features in the evaluation of vasculitis. The size of the subarachnoid hemorrhage (SAH) was decreased on the second postoperative day. The patient's vision and visual fields were improved and she was discharged on the third postoperative day. In outpatient visual field examination on the 18th postoperative day, her visual field was considerably improved from when she was discharged from the hospital, but she continued to have left homonymous quadrianopsia (Fig. 2). Her symptoms were likely due to hemorrhage of the right occipital lobe-visual cortex, and the patient's vision was get back to preoperative vision (0.6).
Go to : 
POVL after non-ophthalmic surgery is rare, with a reported incidence ranging from 0.01 to 0.1% [6]. In 2003, ASA reported 73 cases of POVL occurring after non-ophthalmic surgery (67% in spine surgery, 10% in cardiac bypass surgery) [6]. In 2006, the ASA reported visual loss after spine surgery to occur in less than 0.2% of cases, and the causes were thought to be ischemic optic neuropathy and central retinal artery occlusion [7]. However, visual loss after laparoscopic appendectomy has not been reported previously. A large-scale cohort study was performed with 56,000,000 surgeries in the US [8]. The results estimated that the national incidence of POVL was 8.64/10,000 in cardiac surgery, 1.24/10,000 in colorectal resections and 0.12/10,000 in appendectomies [8]. In another study, POVL was correlated with both the rise in intraocular pressure by Trendelenburg positioning and long operative times [9]. Therefore our case is extremely rare as it occurred post-appendectomy with laparoscopy.
There are other rare case reports, such as a 51-year-old woman who experienced cortical blindness caused by a fat embolism after THR, and another patient who experienced monocular visual loss associated with SAH secondary to ruptured intracranial aneurysmal clipping [10,11]. In 1995, a case of bilateral permanent visual field defect with temporary amaurosis occurred after a massive hemorrhage caused by large vessel injury during a nephrectomy in Korea [12]. In our case, we supposed that the cause of visual disturbance was cerebral hemorrhage. Typically, however, postoperative visual disturbances caused by cerebral hemorrhage are related to brain surgery.
Visual loss by postoperative cerebral hemorrhage is very rare in abdominal surgery without significant bleeding, especially in a low morbidity surgery such as laparoscopic appendectomy. Intraoperative cerebral hemorrhage or vasculitis was initially considered the cause of visual loss of this case. However, as the patient had a stable intraoperative blood pressure without episodes of hypertension and had nonspecific results from a vasculitis examination, it is unlikely that acute hemorrhage or vasculitis occurred during the case. The patient reported that she had a severe headache following an airplane trip two weeks before the operation. This allows us to speculate that a cerebral hemorrhage may have been present before the operation that was possibly aggravated by intraoperative or anesthetic stress. Since blood pressure is usually briefly increased immediately after tracheal intubation and extubation, and blood pressure is only measured at three to five minute intervals, our patient's maximum blood pressure may have been missed, although her systolic blood pressure was consistently lower than 150 mmHg after tracheal intubation. Although a possible small change in blood pressure is unlikely to cause hemorrhage in normal cerebral vessels, when a prior cerebral hemorrhage is present, small changes in blood pressure could increase the size and could have exacerbated our patient's visual symptoms and headache. Additionally, the transmission of the increased intraabdominal pressure by insufflating CO2 during laparoscopy to the thorax led to increased intrathoracic, pleural pressure, and central venous pressure, decreased venous return from the brain and increased intracranial pressure [13,14]. Likewise, increased intra-abdominal pressure due to insufflated CO2 and Trendelenburg positioning increased intracranial pressures (ICP) and Intraocular pressures (IOP) in our case. By these mechanisms, we suggest that postoperative cerebral hemorrhage was aggravated and visual loss occurred.
In an emergency operation, such as this one, preoperative evaluation is often simplified and symptoms not associated with the operation are ignored. We came to find out that the patient had nausea and headache prior to treatment, although we were not aware of this before the operation. If sufficient time was available for preoperative evaluation and testing, we could have prevented or easily treated the postoperative complications. Therefore, we propose more thoroughly evaluating patients preoperatively, including detailed history taking before any operation.
In conclusion, we do not know the exact cause of POVL in this case, but we presume that factors increasing ICP and IOP may predispose patients to cerebral hemorrhage and subsequent POVL.
Go to : 
Notes
The 89th Annual Scientific Meeting of the Korean Society of Anesthesiologists, November 2012, Kimdaejung Convention Center, Gwangju, Korea.
Go to : 
References
1. Shmygalev S, Heller AR. Perioperative visual loss after nonocular surgery. Ophthalmologe. 2011; 108:1067–1076. PMID: 22090093.
2. Torossian A, Schmidt J, Schaffartzik W, Wulf H. Loss of vision after non-ophthalmic surgery: systematic review of the literature on incidence, pathogenesis, treatment and prevention. Anaesthesist. 2006; 55:457–464. PMID: 16416143.
3. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010; 4:531–546. PMID: 20596508.
4. Grover V, Jangra K. Perioperative vision loss: A complication to watch out. J Anaesthesiol Clin Pharmacol. 2012; 28:11–16. PMID: 22345938.

5. Chaudhry T, Chamberlain MC, Vila H. Unusual cause of postoperative blindness. Anesthesiology. 2007; 106:869–870. PMID: 17413925.

6. American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Practice advisory for perioperative visual loss associated with spine surgery: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Anesthesiology. 2012; 116:274–285. PMID: 22227790.
7. American Society of Anesthesiologists Task Force on Perioperative Blindness. Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006; 104:1319–1328. PMID: 16732103.
8. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009; 109:1534–1545. PMID: 19713263.

9. Pinkney TD, King AJ, Walter C, Wilson TR, Maxwell-Armstrong C, Acheson AG. Raised intraocular pressure (IOP) and perioperative visual loss in laparoscopic colorectal surgery: a catastrophe waiting to happen? A systematic review of evidence from other surgical specialities. Tech Coloproctol. 2012; 16:331–335. PMID: 22936587.

10. Gelinas JJ, Cherry R, MacDonald SJ. Fat embolism syndrome after cementless total hip arthroplasty. J Arthroplasty. 2000; 15:809–813. PMID: 11021461.

11. Chong CT, Chin KJ, Yip LW, Singh K. Case series: monocular visual loss associated with subarachnoid hemorrhage secondary to ruptured intracranial aneurysms. Can J Anaesth. 2006; 53:684–689. PMID: 16803916.

12. Kim BH, Shyn KH. Temporary amaurosis with persistent visual field defect following acute blood loss. Korean J Ophthalmol. 1995; 9:47–50. PMID: 7674552.

13. De laet I, Citerio G, Malbrain ML. The influence of intraabdominal hypertension on the central nervous system: current insights and clinical recommendations, is it all in the head? Acta Clin Belg Suppl. 2007; 1:89–97. PMID: 17469706.

14. O'Malley C, Cunningham AJ. Physiologic changes during laparoscopy. Anesthesiol Clin North America. 2001; 19:1–19. PMID: 11244911.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download