Abstract
Torsade de pointes (TdP) is an uncommon and specific form of polymorphic ventricular tachycardia, associated with a prolonged QT interval. Prolongation of the QT interval is the most widely recognized electrophysiological abnormality in patients with liver cirrhosis. We observed a case of TdP leading to cardiopulmonary resuscitation after the induction of general anesthesia, in a patient with liver cirrhosis scheduled for emergency cadaveric donor liver transplantation. The patient had mild QT prolongation on preoperative electrocardiography with a corrected QT (QTc) interval of 455 ms. Drugs used in the preoperative period can elongate cardiac repolarization. Sevoflurane and 5-hydroxytryptamine type 3 receptor antagonists such as palonsetron, used during general anesthesia may have triggered further QT prolongation, producing a fatal condition such as TdP. More caution and consideration in selecting drugs for anesthetic management are necessary for liver cirrhosis patients, especially in patients with preoperative QT prolongation.
Torsade de pointes (TdP) is an uncommon and specific form of polymorphic ventricular tachycardia, characterized by a gradual change in the amplitude and twisting of the QRS complex around the isoelectric line. TdP is associated with a prolonged QT interval, which is the most common electrocardiographic (ECG) finding in patients with liver cirrhosis. Abnormal cardiac repolarization may affect a patient's survival during major surgery, including liver transplantation [1]. Our report describes a case of TdP leading to cardiopulmonary resuscitation (CPR) after the induction of general anesthesia in a liver transplantation recipient. The patient had mild corrected QT (QTc) interval prolongation on preoperative ECG. We suggest that sevoflurane and 5-hydroxytryptamine type 3 receptor antagonist used during the induction of anesthesia may have been a triggering cause of TdP in a patient with liver cirrhosis.
A 53-year old male (height 173.6 cm, weight 75.6 kg) was scheduled for emergency cadaveric donor liver transplantation due to hepatitis B virus-related liver cirrhosis and hepatocellular carcinoma. Microvascular angina was diagnosed 2 years earlier with atypical chest pain. Coronary angiography at that time showed no significant stenotic findings. The patient was diagnosed with atrial fibrillation at the same time, and he was started on nicorandile, molsidomine, isosorbide dinitrate, digoxin, furosemide, and spironolactone. An anticoagulant was excluded due to bleeding tendencies. Furthermore, the patient had liver cirrhosis-related diabetes mellitus, portal hypertensive gastropathy, and esophageal varix.
ECG was performed just before surgery and showed atrial fibrillation with a normal heart rate (Fig. 1). The calculated QTc interval was 455 ms. The patient had mild mitral regurgitation and tricuspid regurgitation, but no regional wall motion abnormality was seen on preoperative echocardiography. The calculated left ventricular ejection fraction was 68%, and the mean pulmonary arterial pressure was 24 mmHg. The PT INR was 1.38, total bilirubin was 2.2 mg/dl, platelet count was 48,000 /µl, and albumin was 2.5 g/dl. Other blood test findings were within normal limits.
Without preoperative medication, the patient entered the operating room and his vital signs were measured immediately. His blood pressure (BP) was 117/73 mmHg, heart rate (HR) was 80 beats/min, and his pulse oximetry oxygen saturation (SpO2) was 99%. After preoxygenation with a mask by applying 100% O2 at 6 L/min for a few minutes, palonsetron 0.075 mg, lidocaine 40 mg, and propofol 120 mg were administered intravenously. After the patient lost consciousness, manual assisted ventilation was performed with 100% O2 at 6 L/min and 5 vol% sevoflurane; in addition, rocuronium 50 mg was injected. With full relaxation, the patient's trachea was intubated using a cuffed 7.5 mm endotracheal tube without difficulty. The radial and femoral arteries were cannulated, followed by continuous arterial blood pressure monitoring. A central venous catheter (AVA HF; Edwards Lifesciences, Irvine, CA, USA) was placed at the right internal jugular vein with no resistance or difficulty. A pulmonary artery catheter (Swan-Ganz CCOmbo; Edwards Lifesciences, Irvine, CA, USA) was inserted and pulmonary artery pressure monitoring was started. By ECG, no clinically significant change occurred during insertion of the pulmonary artery catheter. The catheter's position was checked with a chest X-ray. Anesthesia was maintained with 0.7 L/min O2, 1.3 L/min air, and 1.5-4.0 vol% sevoflurane. Initial arterial blood gas analysis (ABGA) revealed normal electrolyte levels and adequate oxygenation. ECG showed atrial fibrillation, but no clinically significant abnormal change was observed.
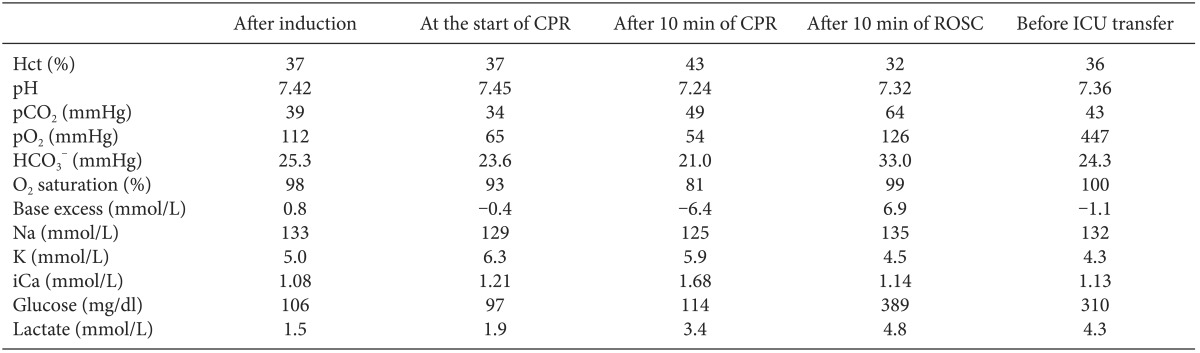
At 50 minutes after the patient's arrival, the surgeon started to prepare for surgery. At that time, sudden-onset large QRS tachycardia (Fig. 2B) appeared, and the femoral and radial arterial waves became flat. The end tidal CO2 (ETCO2) pressure dropped rapidly, from 35 to 13 mmHg. Epinephrine 100 µg and calcium chloride 300 mg were administered twice. One of the surgeons started cardiac compression immediately, and mechanical ventilation was continued with 100% O2. Epinephrine 1 mg was injected intravenously. During CPR, defibrillation was performed four times using 150-200 J, and epinephrine 1 mg was administered intravenously every 3-5 minutes. Amiodarone 300 mg mixed with 100 ml of normal saline was administered. Additionally, magnesium sulfate 2 g and lidocaine 70 mg were injected intravenously. Calcium chloride, regular insulin, and sodium bicarbonate were also administered after checking ABGA (Table 1). During CPR, a bloody secretion started to come out of the endotracheal tube, and the peak inspiratory pressure increased. We repeatedly suctioned the endotracheal tube, and alveolar recruitment via manual bagging was attempted a few times.
After 30 minutes of CPR and the last defibrillation, large QRS tachycardia disappeared and ECG revealed a normal sinus rhythm. Simultaneously, the femoral and radial arterial waves recovered to normal wave forms. The patient's systolic BP was 80-85 mmHg, his HR was 40-45 beats/min, and ETCO2 was 17-19 mmHg. After the return of spontaneous circulation, we loaded 50 µg/kg milrinone intravenously and started continuous infusion of milrinone at 0.5 µg/kg/min and epinephrine at 0.1 µg/kg/min. Regular insulin, sodium bicarbonate, and vasopressin were also administered. The patient's temperature was 34.7℃, and both pupils were dilated without a light reflex.
A few minutes later, the patient's SpO2 increased to 100%, and ETCO2 increased to 28 mmHg. The patient's vital signs stabilized at a HR of 80-85 beats/min, systolic BP over 100 mmHg, and mean BP over 80 mmHg. Without proceeding with the operation, the patient was transferred to the intensive care unit (ICU). The total exposure time to anesthesia was about 110 minutes. 1 L of crystalloid and 400 ml of colloid were infused, and the patient's urine output was 30 ml during anesthesia. On arrival at the ICU, the patient's BP was 98/62/76 mmHg, his SpO2 was 100%, and ECG showed atrial fibrillation with a HR of 80 beats/min. The patient's pupil reflex returned to a normal response. Electrolytes and cardiac enzymes were evaluated. Neither hypokalemia nor hypocalcemia was detected, and all electrolyte values were within normal levels. The patient's CK was 106 IU/L, CK-MB was 2.6 ng/ml, and Troponin I was slightly elevated to 0.10 ng/ml. Bedside echocardiography revealed no regional wall motion abnormality or evidence of myocardial ischemia.
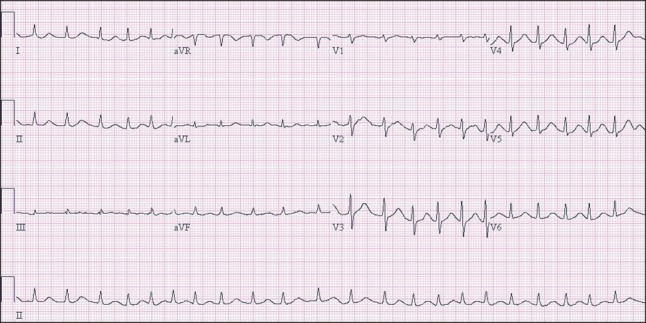
On the second day of ICU admission, pulse-less large QRS tachycardia appeared twice. Both events were terminated after applying defibrillation using 150 J and cardiac compression for 2 minutes. Metabolic acidosis, intravascular volume loss, and pulmonary edema were aggravated and the patient's BP and SpO2 continued to decrease. On the fourth day in the ICU, the patient expired. ECG showed a marked prolonged QTc interval (Fig. 3). Nevertheless, cardiac echocardiography performed on the day of the patient's expiry showed normal findings. No regional wall motion abnormality was observed, and the left ventricular wall thickness was normal with a calculated ejection fraction of 56%. The patient's systolic pulmonary arterial pressure was 33 mmHg.
Long QT syndrome is a congenital or acquired disorder of cardiac ion channels, characterized by heterogeneity in cellular repolarization and precipitation of tachyarrhythmias [2]. The character of this syndrome is a marked prolongation of ventricular repolarization, including QT interval prolongation and T wave abnormalities leading to ventricular tachycardia, such as TdP and ventricular fibrillation [3]. Although a normal value has not been established, QTc intervals longer than 440 ms are generally considered to indicate prolonged QT. As in the present case, a prolonged QTc interval is related to sudden death and poor survival in patients with various diseases [3,4].
Prolongation of the QT interval is the most widely recognized electrophysiological abnormality in liver cirrhosis and has been observed in about half of patients with liver cirrhosis [1,5,6]. Several investigations have reported that QT prolongation increases with the severity of liver disease, but it can also occur in patients with well-compensated cirrhosis [1,5,6]. Bal and Thuluvath [5] demonstrated an association between liver cirrhosis and QT prolongation, showing that a worsening of the Child-Pugh score was associated with further prolongation of the QT interval. Some investigators have demonstrated that the QT interval is an additional prognostic factor for life expectancy [7]. The present patient had Child-Pugh class C liver cirrhosis with numerous accompanying complications. The QTc calculated by preoperative ECG was 455 ms.
Several drugs used in the perioperative period could induce the lengthening of cardiac repolarization [8,9]. In many ECG studies, sevoflurane has been shown to lengthen the QT interval during the induction of inhalational anesthesia [8,10]. 5-Hydroxytryptamine type 3 receptor antagonists commonly used to prevent or treat postoperative nausea and vomiting, have also been demonstrated to induce QT prolongation [11]. In our case, palonsetron was administered during the induction of anesthesia and sevoflurane was used to maintain anesthesia. Palonsetron is a 5-hydroxytryptamine type 3 receptor antagonist. Thus, palonsetron may have been an aggravating factor in QT prolongation, although its effect on the QT interval has not yet been specifically investigated. A few seconds before the occurrence of TdP, the QTc interval was prolonged to 492 ms (Fig. 2A). TdP only occurred before any surgical procedure, so the anesthetic agents used during induction are suspected to be the most probable cause. That no sympathetic hyperactivity was seen before the occurrence of TdP and ABGA performed just before the initiation of TdP was within normal limits support this. Although the patient had diagnosed microvascular angina, preoperative ECG showed only atrial fibrillation and no evidence of myocardial ischemia. Preoperative cardiac echocardiography and coronary angiography revealed normal findings. Furthermore, cardiac echocardiography and cardiac enzymes evaluated after CPR did not indicate any ischemic insult. Atrial fibrillation may have been a contributing factor in the occurrence of TdP; however it is hard to ascribe myocardial ischemia as a major cause of TdP.
We undertook a retrospective analysis of the ECG waveform during anesthesia and found that TdP was the initiating ECG form during the patient's arrhythmia and arrest. Ventricular premature beats occurred, and the R-on-T phenomenon preceded TdP (Figs. 2C and 2D). Unfortunately, we did not know that TdP was the first ECG form at the time of arrest; thus, we managed the patient's arrhythmia according to standard CPR guidelines. We administered amiodarone intravenously, which may have aggravated the QT prolongation. Amiodarone is known to prolong the QT interval, but with a low incidence of proarrhythmic events [12]. Nevertheless, QT prolongation with subsequent TdP after amiodarone therapy potentially resulting in ventricular fibrillation has been repeatedly reported [13].
Preoperative management of patients with long QT syndrome should be performed in a comfortable environment. Midazolam and fentanyl can be used to prevent anxiolysis without complications [14]. The use of drugs known to prolong the QT interval should be avoided as far as possible. Perioperative infusion of magnesium sulfate is recommended as prophylaxis against TdP [14]. A defibrillator and transvenous pacing wires and leads should be prepared for prompt use [2,15]. Hypothermia should be avoided since it may prolong the QT interval, possibly through the delayed recovery of inactivated sodium channels [2,14,15].
Magnesium sulfate is the treatment of choice for TdP [14]. Serum electrolyte levels should be checked and corrected within normal limits. Temporary transvenous pacing can be effective in some patients. Defibrillation and CPR may be necessary and lidocaine may be useful in terminating arrhythmia [14].
In conclusion, patients with liver cirrhosis may have preoperative QT prolongation, but insufficient awareness of this electrophysiological abnormality is common. Sevoflurane and 5-hydroxytryptamine type 3 receptor antagonists used during anesthesia can trigger further QT prolongation. This can lead to fatal conditions, including TdP, as in the present case. More caution and consideration when selecting drugs and inhalation agents during anesthetic management are necessary for patients with liver cirrhosis, especially patients with preoperative QT prolongation. In particular, 5-hydroxytryptamine type 3 receptor antagonists should be avoided and replaced with other anti-emetic drugs, and sevoflurane should be used with caution. Vital signs and ECG should be carefully monitored, and the QTc should be evaluated continuously during anesthesia if possible. Furthermore, in vulnerable patients with preoperative QT prolongation, TdP should be considered as a possible cause when fatal arrhythmia occurs. Deterioration in a patient's condition due to an inappropriate medication such as amiodarone can be prevented by scrupulous attention and an adequate diagnosis. Accurate and expeditious management is also required.
References
1. Zambruni A, Trevisani F, Caraceni P, Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol. 2006; 44:994–1002. PMID: 16510203.

2. Staikou C, Chondrogiannis K, Mani A. Perioperative management of hereditary arrhythmogenic syndromes. Br J Anaesth. 2012; 108:730–744. PMID: 22499746.

3. Jackman WM, Friday KJ, Anderson JL, Aliot EM, Clark M, Lazzara R. The long QT syndromes: a critical review, new clinical observations and a unifying hypothesis. Prog Cardiovasc Dis. 1988; 31:115–172. PMID: 3047813.

4. Elming H, Holm E, Jun L, Torp-Pedersen C, Køber L, Kircshoff M, et al. The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J. 1998; 19:1391–1400. PMID: 9792266.

5. Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003; 23:243–248. PMID: 12895263.

6. Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998; 27:28–34. PMID: 9425913.

7. Kosar F, Ates F, Sahin I, Karincaoglu M, Yildirim B. QT interval analysis in patients with chronic liver disease: a prospective study. Angiology. 2007; 58:218–224. PMID: 17495272.

8. Yildirim H, Adanir T, Atay A, Katircioğlu K, Savaci S. The effects of sevoflurane, isoflurane and desflurane on QT interval of the ECG. Eur J Anaesthesiol. 2004; 21:566–570. PMID: 15318470.

9. Schmeling WT, Warltier DC, McDonald DJ, Madsen KE, Atlee JL, Kampine JP. Prolongation of the QT interval by enflurane, isoflurane, and halothane in humans. Anesth Analg. 1991; 72:137–144. PMID: 1898684.

10. Kleinsasser A, Kuenszberg E, Loeckinger A, Keller C, Hoermann C, Lindner KH, et al. Sevoflurane, but not propofol, significantly prolongs the Q-T interval. Anesth Analg. 2000; 90:25–27. PMID: 10624970.

11. Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005; 102:1094–1100. PMID: 15915019.

12. Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated proarrhythmic effects. A review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994; 121:529–535. PMID: 8067651.

13. Schrickel J, Bielik H, Yang A, Schwab JO, Shlevkov N, Schimpf R, et al. Amiodarone-associated "torsade de pointes". Relevance of concomitant cardiovascular medication in a patient with atrial fibrillation and structural heart disease. Z Kardiol. 2003; 92:889–892. PMID: 14579055.
14. Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for patients with congenital long QT syndrome. Anesthesiology. 2005; 102:204–210. PMID: 15618804.

15. Katz RI. Some points regarding anesthesia for patients with congenital long QT syndrome. Anesthesiology. 2005; 103:1315–1316. PMID: 16306750.

Fig. 1
Preoperative electrocardiography. The calculated corrected QT interval from lead II is 455 ms.
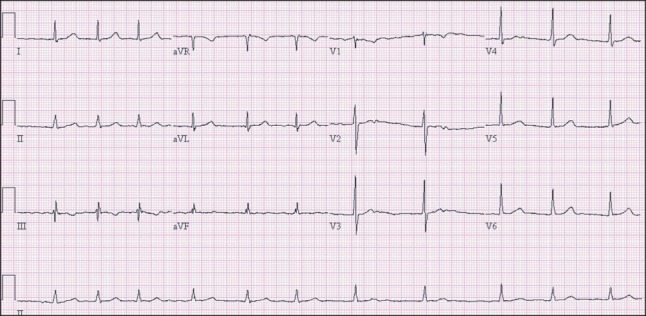
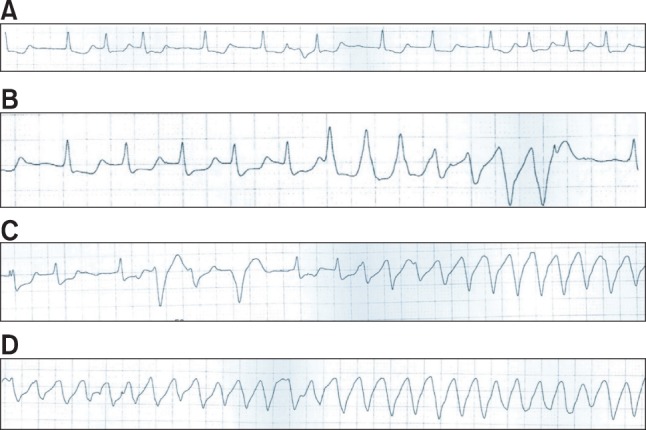
Fig. 2
Changes observed in lead II by electrocardiography (ECG) during anesthesia. (A) ECG after anesthetic induction (QTc = 492 ms) showed QTc interval prolongation. (B) At 50 min after the patient's arrival, large QRS tachycardia appeared (QTc = 480-490 ms). (C) The R-on-T phenomenon and ventricular premature beats were observed during the occurrence of large QRS tachycardia. (D) During cardiac arrest, ECG demonstrated torsade de pointes. ECG: electrocardiography, QTc: corrected QT interval.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download