Abstract
Systemic capillary leak syndrome (SCLS) is very rare and lethal disease and only 150 cases have been reported after the first publication of its report in 1960 by Clarkson. SCLS is characterized by hemoconcentation and hypoalbuminemia caused by reversible plasma extravasation. Its mechanism is unknown, but transient dysfunction of the endothelium is the most suspected cause and trigger of this event may cause immunologic disarrangement. After recovery of endothelial function, fluid injected during the shock period is redistributed and can cause severe pulmonary edema. SCLS should be considered in patients with acute and severe hypotension with hemoconcentration and hypoalbuminemia without obvious cardiac dysfunction. Especially we should take into account the possibility of SCLS if fluid replacement does not work or the shock state is aggravated despite aggressive fluid resuscitation and vasopressor administration. SCLS itself is a very rare disease; furthermore, SCLS that develops during well-controlled surgery is even more rare. So we report this case with review of the literature.
Go to : 
Systemic capillary leak syndrome (SCLS) was first reported by Clarkson in 1960 [1]. It is a rare and fatal disease with less than 150 reported cases and is characterized by hemoconcentration and hypoalbuminemia caused by reversible plasma extravasation, and it is presumed that the cause of this disease is an immunological disorder [2,3]. Many cases of SCLS have occurred suddenly in healthy persons [1,4].
In many studies, it is presumed that endothelial dysfunction in SCLS is caused by apoptosis of endothelial cells or endothelial contraction [1,2,4]. In a study investigating the mechanism of lung injury in ARDS, it was shown that inflammatory mediators were deeply involved in apoptosis of endothelial cells and diffuse capillary leak [5,6].
This case was clinically diagnosed as SCLS because of hypotension, pulmonary edema and hemoconcentration that occurred suddenly during elective surgery, and it is a very rare disease, and also the case developed during surgery, which is even rarer. Hence, we report this case.
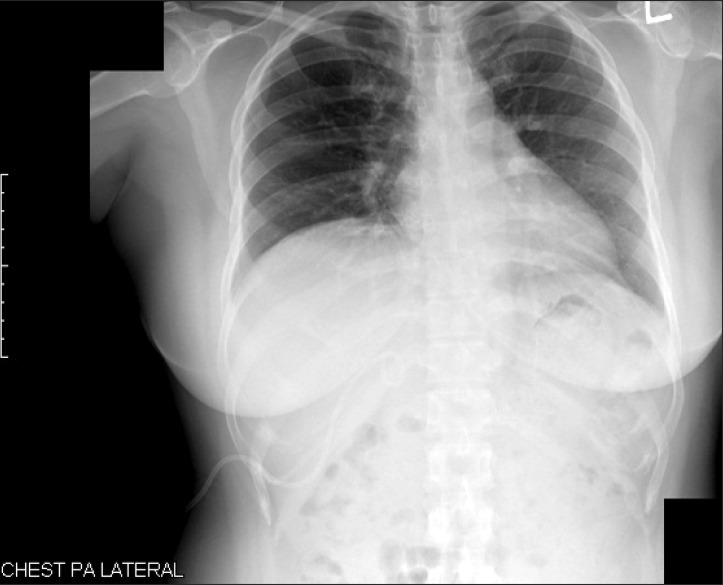
A female patient of 41 years of age with a height of 152.5 cm and a weight of 67.8 kg came to the emergency room because of pruritus as a major symptom and she had no other symptom besides general pruritus at that time. The blood test, urinalysis, chest X-ray (Fig. 1), and electrocardiogram (ECG) were conducted in the emergency room. The results showed aspartate aminotransferase was 70 IU/L, alanine aminotransferase 60 IU/L, alkaline phosphatase 1,249 IU/L, bilirubin 2.6 mg/dl, and gamma glutamyl transferase 1,218 IU/L, so obstructive jaundice was diagnosed. Because of the obstructive jaundice, computed tomography (CT) and magnetic resonance cholangio-pancreatograpy were additionally performed. In the result, distal common bile duct cancer was confirmed, so it was decided to perform pylorus preserving pancreatoduodenectomy. Before surgery, the test result showed normal chest radiography (Fig. 1), normal ECG, hemoglobin 12.7 g/dl, hematocrit 39.8%, white blood cell 19,600 /mm3, platelet 309,000 /mm3, and albumin 3.5 g/dl, with no unusual finding and the diabetes was well controlled in the range of glucose of 100-140 mg/dl.
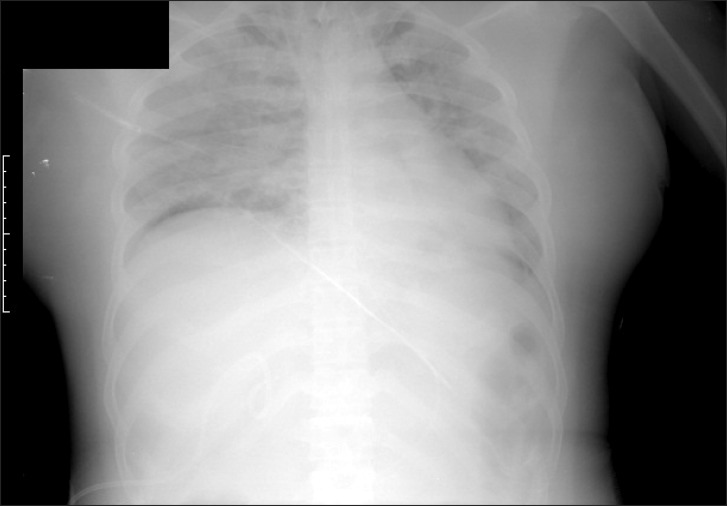
For general anesthesia, thiopental sodium 300 mg and vecuronium 8 mg for the endotracheal intubation were intravenously injected. Tracheal intubation was conducted with a single lumen tube of 7.5 mm in its internal diameter and intubation easily proceeded on the first try. The maintenance of anesthesia was done by desflurane 5.0-6.0%, 50% nitrous oxide and oxygen. The patient was maintained with a tidal volume of 8 ml/kg, and a respiratory rate of 10 breaths/min by the volume controlled mode. ECG, bispectral index (BIS), and pulse oximetry were performed, and a 20-gauge catheter was inserted in the right radial artery for continuous arterial pressure monitoring and a central venous catheter was inserted in the right internal jugular vein for the measurement of central venous pressure and for transfusion of fluids. The patient's vital signs immediately after surgery were stable (systolic/diastolic blood pressure 100-130/60-80 mmHg, heart rate 83-97 beats/min, BIS 45-50, central venous pressure 10 mmHg, and pulse oximetry saturation 100%). In the patient, end-tidal carbon dioxide (EtCO2) was rapidly reduced and then disappeared, and hypotension (50-60/20-30 mmHg), bradycardia (30-40 beats/min), central venous pressure 3 mmHg, rapidly decreased BIS value, and oxygen saturation of 88% were observed without sudden massive loss of blood or unusual conditions during surgery over four hours after starting the operation, so FiO2 was increased by 1.0 and ephedrine 10 mg, phenylephrine 100 µg, and epinephrine 450 µg were intravenously injected. However, her vital signs were not improved and arterial pressure rapidly decreased and then disappeared. Epinephrine 3 mg and atropine 1 mg were injected and cardiac massage was conducted for 20 minutes because of acrostic electrical activity on ECG. After that, arterial blood pressure was 150/93 mmHg, and pulse 140 beats/min, and an oxygen saturation of 100% was confirmed. BIS value did not recover after it was reduced during cardiopulmonary resuscitation. The result of the arterial blood gas examination at that time showed hemoglobin was 8.2 mg/dl, hematocrit 24%, PaO2 83 mmHg, PaCO2 51 mmHg, pH 7.13, ionized calcium 3.05 mg/dl, and glucose 254 mg/dl. Considering that the arterial blood gas examination was conducted after injecting 1,500 ml fluid within 30 minutes with cardiopulmonary resuscitation, it was determined that hemodilution was present rather than decreased hemoglobin caused by loss of blood. Calcium chloride 300 mg to prevent myocardial contractility reduction caused by hypocalcaemia, sodium bicarbonate 80 mEq for acidosis, and regular insulin 5 units for glucose control were intravenously injected. Colloid 1,000 ml, crystalloid 3,000 ml, and 2-pint packed red blood cells were injected for three hours after the occurrence of the hypotension. Then, the result of the arterial blood gas examination showed hemoglobin was 16.7 mg/dl, hematocrit 49%, PaO2 89 mmHg, PaCO2 38 mmHg, pH 7.37, ionized calcium 3.45 mg/dl, glucose 187 mg/dl, and so hemoconcentration was observed. Transesophageal ultrasonography was conducted to confirm cardiac functions during surgery and to provide fluids, but no unusual finding was found. After cardiopulmonary resuscitation, dopamine 5-10 µg/kg/min was injected to maintain the arterial blood pressure at 120-80/80-40 mmHg and body temperature at 34-36℃. The anesthetic duration was 9 hours and 45 minutes and the surgical time was 8 hours and 35 minutes. Estimated blood loss was 1,800 ml and urine output was 2,670 ml, and the injected fluid and blood included crystalloid 5,600 ml, colloid 1,000 ml, and packed red blood cell 2 pints. On chest radiography conducted after surgery, pulmonary edema was observed without cardiomegaly in the bilateral pulmonary area. Her weight was 72.3 kg, and this increased by approximately 5 kg after she was transferred to the intensive care unit (Fig. 2).
The patient was intravenously injected by dopamine 10 µg/kg/min with airway intubation under anesthesia after surgery and transferred to the surgical intensive care unit. After surgery, the result showed hemoglobin 18.1 g/d, hematocrit 51.9%, white blood cell 23,600 /mm3, platelet 363,000 /mm3, albumin 1.6 g/dl, and so hemoconcentration and hypoalbuminemia were confirmed. Therefore, SCLS was presumptively diagnosed. Mechanical ventilation care was performed with the pressure support mode because of the pulmonary edema. Theophylline 400 mg/day and solucortef 300 mg/day were intravenously injected to improve systemic capillary leak syndrome, and dopamine, dobutamine, and norepinephrine were also injected to improve hypotension. Hemoglobin was 11.8 g/dl and hematocrit was 35.1% at Day 1 after surgery and albumin was 3.3 g/dl at Day 3, and so these values had normalized. Pulmonary edema was improved at Day 13 after surgery. The mental status of the patient was semicomatose and deep drowsy and it was not improved. On diffusion magnetic resonance imaging conducted after surgery, hypoxic brain damage was confirmed. On electroencephalography, severe diffuse cerebral dysfunction was confirmed. Tracheostomy was conducted eight months after the operation and since then SICU conservative care has been in progress.
Go to : 
In SCLS, sudden shock and edema develop due to plasma extravasation caused by endothelial dysfunction. It may be accompanied by asthenia universalis, fever, emesis, abdominal pain, diarrhea, etc. and it may occur suddenly without a prodrome [1,2,3]. Shock and edema can continue for several days and the flow of fluid from the four limbs and trunk is found, but visceral edema, such as pleural exudate and pericardial exudates, does not typically occur [4]. As the function of the capillary barrier recovers, symptoms improve. In this period, fluid injected to treat shock is rapidly redistributed, so pulmonary edema occurs and this can cause death [4]. Therefore, the amount of fluid injected to prevent damage caused by organ ischemia during hypovolemic shock must be very carefully monitored. Excessive supply of fluid can cause compartment syndrome, rhabdomyolysis, or renal failure as well as pulmonary edema [4].
In the case of hypotension, hemoconcentration, and hypoalbuminemia without any special cause to induce shock present, SCLS can be diagnosed. Especially, the co-occurrence of hypotension and hemoconcentration enables clinicians to infer that the hypovolemia was caused by destruction of vascular endothelial cells. In the case of acute hypotension and hemoconcentration not reacting to aggressive fluid supplement or vasoconstrictors without a decline of cardiac function, SCLS should be considered. Complete blood count, blood and urine culture, serum immunofixation electrophoresis, serum albumin level, serum tryptase, chest radiography, electrocardiography, and echocardiography are required for the diagnosis in the presence of symptoms. When IgG kappa monoclonal gammopathy is confirmed without the possibility of multiple myeloma, it can be an accessory standard for the diagnosis of SCLS, but it is not essential for diagnosis [2,4,7]. Intravenous injection of theophylline decreases the duration and severity of the acute phase, and hypertensors and fluid are required as appropriate. Colloids remaining in the intravascular space are preferred for the fluid, and fluids should keep central venous pressure only at the level that prevents damage caused by hypoperfusion, so that compartment syndrome or rhabdomyolysis can be prevented [2,4]. In the case of phlebothrombosis, anticoagulant therapy should be conducted. In the case of severe pulmonary edema, the use of a respirator should be considered and the symptoms can be improved by intravenous high-dose immunoglobulin, but the mechanism for this is unknown [8,9].
Coronary artery spasm, pulmonary thromboembolism, inferior vena cava compression syndrome, idiopathic anaphylaxis, sepsis, acute respiratory distress syndrome (ARDS) due to aspiration, systemic inflammation due to surgical trauma that can induce a sudden drop of blood pressure, loss of EtCO2, and bilateral pulmonary edema were suspected, but excluded by the reasons that follow. Transesophageal ultrasonography during surgery was conducted to confirm whether the cause was heart failure due to coronary artery spasm occurring during surgery. However, the motility of the cardiac walls was normal, and ergonovine, acetylcholine, and vasoconstrictors that provoke coronary spasm had not been injected, and also hyperventilation was not observed [10,11].
Sudden loss of EtCO2 and hypotension occurred, so thromboembolism was suspected, but findings of right ventricular malfunction due to pulmonary embolism, such as dilatation of the right atrium and the right ventricle, and left shift of the interventricular septum, were not observed on transesophageal ultrasonography during surgery [12].
The possibility of compression of the inferior vena cava during surgery or compression caused by a pathological process was confirmed by an operator, but no unusual conditions were found.
Idiopathic anaphylaxis can induce acute hypotension and angioedema, but the angioedema is localized in the upper airway, with a normal albumin level, and a great reaction to epinephrine. Therefore, it can be excluded [13].
In the case of sepsis, hypotension and edema occurred, so the syndrome may look similar to sepsis. However, the albumin level was not decreased and there was no signs to suspect infection before the development of sudden hypotension. Therefore, sepsis can be excluded [4].
ARDS due to aspiration during anesthesia can be excluded because proper fasting was conducted for the elective surgery, and intubation was not repeatedly tried, and aspiration of gastric contents was not observed. Systemic inflammation caused by surgical trauma was not completely excluded because proinflammatory cytokines were not evaluated [6].
In the case of exclusion of multiple myeloma, an accessory standard for diagnosis of SCLS, serum electrophoresis, serum IgG, IgA, IgM, IgE level were confirmed to verify monoclonal gammopathy (post op 1 day: IgG: 295 mg/dl, post op 2 days: IgG: 406.5 mg/dl, IgA: 98.8 mg/dl, IgM: 40 mg/dl, post op 3 days: IgG: 416.7 mg/dl, normal value of IgG: 694-1,618 mg/dl, IgA: 60-263 mg/dl, IgM: 60-263 mg/dl, serum electrophoresis result: no abnormal band), but the results were not confirmed as monoclonal gammopathy. Many SCLS patients have monoclonal gammopathy, but some studies have expressed doubt that paraprotein is a direct cause of SCLS [2]. This case did not accord with monoclonal gammopathy which is an accessory standard for the definite diagnosis of multiple myeloma, but other diseases were excluded. Therefore, it was presumptively diagnosed as SCLS [1,2,3,4,7,8].
In this case, it was decided that hypoxic brain damage had been caused by inappropriate perfusion of the brain during cardiopulmonary resuscitation, so the use of a respirator in the intensive care unit was unavoidable because of the absence of appropriate spontaneous respiration.
We need to be aware that SCLS can occur in patients without underlying diseases or history of a previous SCLS attack during elective surgery. When sudden bilateral pulmonary edema and severe hypotension not reacting to hypertensors occur, evaluation and treatment are required to be performed with consideration of the possibility of SCLS in the case where the cause is unclear.
Go to : 
References
1. Clarkson B, Thompson D, Horwith M, Luckey EH. Cyclical edema and shock due to increased capillary permeability. Am J Med. 1960; 29:193–216. PMID: 13693909.

2. Amoura Z, Papo T, Ninet J, Hatron PY, Guillaumie J, Piette AM, et al. Systemic capillary leak syndrome: report on 13 patients with special focus on course and treatment. Am J Med. 1997; 103:514–519. PMID: 9428835.
3. Dhir V, Arya V, Malav IC, Suryanarayanan BS, Gupta R, Dey AB. Idiopathic systemic capillary leak syndrome (SCLS): Case report and systemic review of case reported in the 16 years. Intern Med. 2007; 46:899–904. PMID: 17575386.
4. Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010; 153:90–98. PMID: 20643990.

5. Salzer WL, McCall CE. Primed stimulation of isolated perfused rabbit lung by endotoxin and platelet activating factor induced enhanced production of thrombixane and lung injury. J Clin Invest. 1990; 85:1135–1143. PMID: 2318970.
6. Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H. Upregulation of two death pathways of Perforin/Granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000; 161:237–243. PMID: 10619826.

7. Kawabe S, Saeki T, Yamazaki H, Nagai M, Aoyagi R, Miyamura S. Systemic capillary leak syndrome. Intern Med. 2002; 41:211–215. PMID: 11929183.

8. Lambert M, Launay D, Hachulla E, Morell-Dubois S, Soland V, Queyrel V, et al. High dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit Care Med. 2008; 36:2184–2187. PMID: 18552679.
9. Atkinson JP, Waldmann TA, Stein SF, Gelfand JA, Macdonald WJ, Heck LW, et al. Systemic capillary leak syndrome and monoclonal IgG gammopathy : studies in a sixth patient and a review of the literature. Medicine (Baltimore). 1977; 56:225–239. PMID: 870792.
10. Cannon CP, Braunwald E. Unstable angina and non-ST-elevation myocardial infarction. In : Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jamson JL, editors. Harrison's principles of internal medicine. 16th ed. New York: McGraw-Hill medical publishing division;2005. p. 1444–1448.
11. Austen KF. Allergies, anaphylaxis, and systemic mastocytosis. In : Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jamson JL, editors. Harrison's principles of internal medicine. 16th ed. New York: McGraw-Hill medical publishing division;2005. p. 1949–1951.
12. Jang IS, Kim HT, An SK, Kwon YE, Lee JH. Pulmonary thromboembolism occurred immediately after leg elevation under induction of general anesthesia in a patient with femur fracture. Anesth Pain Med. 2009; 4:129–132.
13. Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM. Sign of myocardial ischemia after injection of oxytocine: a randomized double -blind comparison of oxytocine and methylergometrine during Caesarean section. Br J Anaesth. 2008; 100:683–689. PMID: 18385263.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download