Abstract
Perioperative ischemic stroke is an uncommon event associated with significant morbidity and mortality. The complexity of the surgical procedure and surgery induced hypercoagulable status also influence the incidence of stroke. The management of stroke involves a decision regarding the quickest suitable revascularization method. Endovascular mechanical thrombectomy, such as intra-arterial mechanical thrombectomy (IAMT), can restore vascular patency of the vessels, providing an alternative or synergistic method to restore blood flow. Although, there are no recommended treatment guidelines, IAMT is eligible to be a treatment of choice for perioperative ischemic stroke. We experienced a case of a patient who demonstrated hemiplegia and aphasia, the early symptom of acute ischemic stroke, in the post-anesthesia care unit and performed IAMT successfully. Thus we report the case with a review of the relevant literature.
Perioperative ischemic stroke is an uncommon event associated with significant morbidity and mortality. Higher risks such as a stroke are usually associated with major cardiovascular surgeries. However, the risk of stroke could be associated with general surgery [1].
Age ≥ 62 year, history of myocardial infarction within 6 months before surgery, acute renal failure, history of stroke, dialysis, hypertension, history of transient ischemic attack, chronic obstructive pulmonary disease, current tobacco use, and body mass index 35-40 kg/m2 are known as independent predictors of stroke [2]. The complexity of the surgical procedure and surgery induced hypercoagulable status also influence the incidence of stroke [2]. The management of stroke involves a decision regarding the quickest suitable revascularization metod. For a long time, the gold standard treatment t for acute ischemic stroke (AIS) has been intravenous recombinant tissue plasminogen activator (rtPA) and general intensive care measures. Endovascular mechanical thrombectomy, such as intra-arterial mechanical thrombectomy (IAMT), can restore vascular patency of the vessels, providing an alternative or synergistic method to restore blood flow [3]. Although, there are no recommended treatment guidelines, IAMT is eligible to be a treatment of choice for perioperative AIS.
We experienced a case of a patient who demonstrated hemiplegia and aphasia, the early symptom of AIS, in the postanesthesia care unit, and performed IAMT successfully. Thus we report the case with a review of the relevant literature.
A 65-year-old man, who was 172 cm tall and weighed 76 kg, underwent arthroscopic rotator cuff repair for left shoulder rotator cuff tear. His medical history was significant for non-insulin dependent diabetes mellitus and stable angina, with medications to control those conditions (aspirin, oral hypoglycemic agent). Aspirin (100 mg/day) was discontinued 7 days before surgery. Results of all preoperative laboratory tests including electrocardiogram, chest X-ray, blood examination and urine examination were within normal limits. Preoperative prothrombin time and activated partial thromboplastin time were 13.4 and, 29.9, respectively. Platelet function tests were not performed.
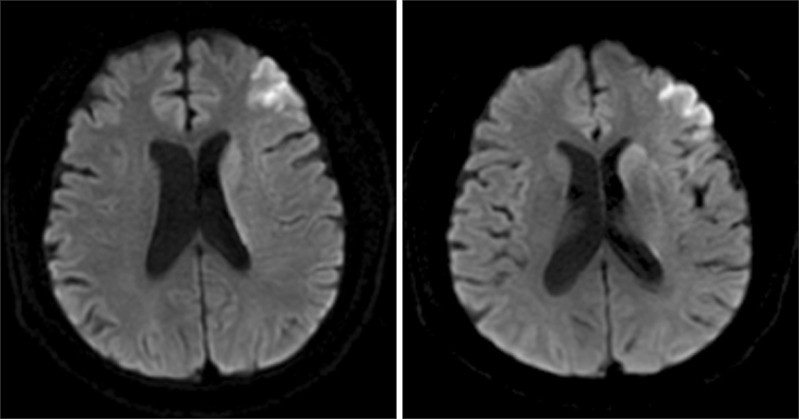
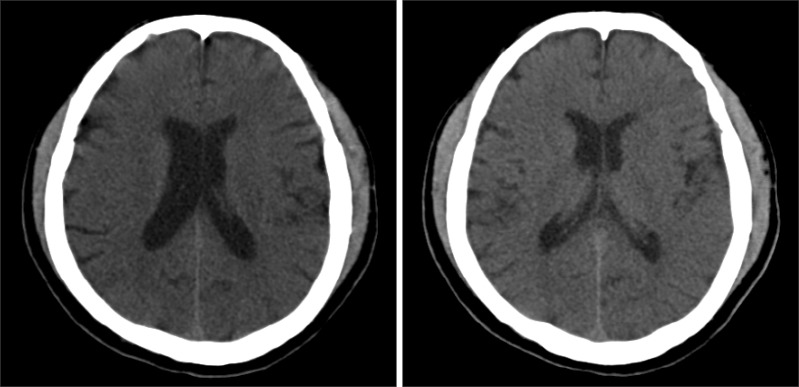
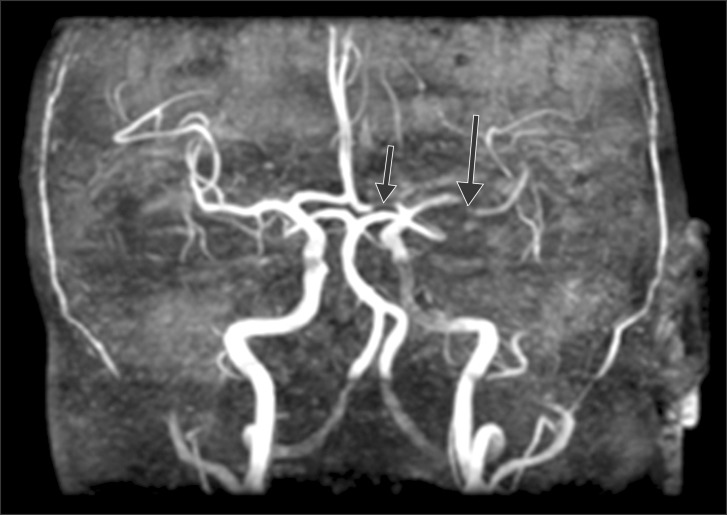
Intraoperative monitorings included non invasive blood pressure, three lead electrocardiogram, and pulse oximetry. After arrival in the operating room, he patients vital signs were a blood pressure of 150/82 mmHg, a heart rate of 98 /min, a respiration rate of 20 /min and SpO2 of 99%. Infusion of remifentanil was started at the rate of 1 µg/kg/min. Lidocaine 80 mg and propofol 150 mg were then injected for induction of anesthesia. After loss of consciousness, rocuronium 60 mg was administered intravenously with mask ventilation and the patient was intubated endotracheally. During the surgery, the patient was maintained in a semi upright beach chair position. A blood pressure cuff was in place around the patient's right arm, and the mean blood pressure was maintained above 80 mmHg, considering the patient's position. Remifentanil was infused at the rate of 0.1-0.3 µg/kg/min to minimize hemodynamic changes, while at the same time anesthesia was maintained with sevoflurane and 40% oxygen. Arthroscopic irrigation was performed with 42,000 ml of saline and epinephrine 12 mg. At the end of the operation, pyridostigmine 15 mg and glycopyrrolate 0.4 mg were intravenously injected after the patient had recovered spontaneous breathing and the patient was then extubated. The total operation time was 3 hours, 50 minutes, and anesthesia time was 4 hours, 55 minutes. When the patient arrived at the post-anesthesia care unit, his vital signs were a blood pressure of 120/60 mmHg, a heart rate of 80 /min, a respiration rate of 15 /min and SpO2 of 100% with an oxygen mask at 5 L/min. The patient was at slightly sedated status in response to verbal commands. After 30 minutes, hemiplegia (motor grade I) and aphasia were observed. Immediately, after checking the blood sugar level (170 mg/dl), brain computed tomography (CT) was taken for differential diagnosis of acute cerebral infarction and acute cerebral hemorrhage. The result of the brain CT shows no intracranial hemorrhage (Fig. 1). Diffusion brain magnetic resonance imaging shows two focal hyper acute infarcts in the left anterior frontal lobe and basal ganglia, respectively (Fig. 2). Brain magnetic resonance angiography shows a significant narrowing at the left A1 and M1 segment occlusion of the left middle cerebral artery superior division (Fig. 3). At first, we considered administering intravenous rtPA, but decided to perform IAMT instead due to the risk of hemorrhage in the immediate postoperative period. The patient was sedated with midazolam due to confused mental status. Thrombus at A1 and the M1 branch of the middle cerebral artery were removed successfully (Fig. 4). The IAMT procedure took about 50 minutes, and it was begun 3 hours and 30 minutes after detection of AIS symptoms. Motor grade was changed from I to II shortly after the procedure was finished. The patient was observed in the intensive care unit for 7 days and showed improved mental status and motor function. Follow up brain CT and transthoracic echocardiography were done, and there were no significant findings. After one month, the patient was discharged without sequelae.
Stroke is an important cause of morbidity and mortality in the perioperative period. During the perioperative period, the pro-atherogenic and pro-thrombotic effects of the inflammatory response amplify the mortality of stroke [4]. The incidence of perioperative stroke is 0.1% and it is associated with an 8-fold increase in perioperative mortality within 30 days [2].
The rate of thromboembolic events after arthroscopic procedures on the shoulder is considered low. The main thromboembolic phenomena associated with shoulder arthroscopy are deep vein thromboses or pulmonary thromboembolisms [5]. Arterial thromboembolisms are not a common complication.
This patient had just two risk factors (age > 62, hypertension), and he was in a low cerebral ischemic risk category. He had discontinued aspirin 7 days prior to the surgery under cardiology consultation. However, shoulder arthroscopic surgery is not a major surgery that may cause bleeding risks with increased mortality. Aspirin for secondary cardiovascular prevention increases neither the level of the severity of bleeding complications nor the perioperative mortality due to bleeding complications [6]. Withdrawal of low dose aspirin (< 200 mg/day) may cause platelet rebound phenomenon. Retrospective investigations revealed that withdrawal of low-dose aspirin for secondary prevention precedes up to 10.2% of acute cardiovascular syndromes. The time interval between discontinuation and acute cerebral events was 14.3 ± 11.3 days [6]. Thus, aspirin should only be discontinued perioperatively, if aspirin's known or assumed perioperative bleeding risks and their sequels are expected to be similar to or more severe than the observed cardiovascular risks after aspirin withdrawal (myocardial infarction, stroke, peripheral vascular occlusion, or cardiovascular death).
While neither preoperative platelet function tests nor analysis of urinary metabolites of thromboxane A2 and prostacycline were done in this case, the influence of platelet rebound could not be ruled out.
Moreover, there is a possibility of increasing ischemic risk from decreased cerebral blood flow due to the intraoperative beach chair position. Shoulder arthroscopy can be performed with the patient in either the lateral decubitus or beach chair position, but ischemic stroke is more common in the beach chair position [7]. In this case, invasive arterial blood pressure monitoring was not performed, but mean arterial blood pressure was maintained above 80 mmHg, based on the height of the position of the blood pressure cuff on the right arm from the external auditory meatus [8].
As AIS is stroke within 4.5 hours of symptom onset, early detection and proper management are very important to patient prognosis [3].
This patient's symptoms were detected relatively early in the post-anesthesia care unit, and the stroke clinical pathway was immediately activated. The evaluation and appropriate management for stroke were not delayed.
The initial evaluation of a stroke patient is similar to that of other critically ill patients. However, a second assessment of neurological deficits and comorbidities, following rapidly behind the initial patient assessment, is also needed in the evaluation of a stroke. In this period, it is essential to exclude stroke mimics, such as conversion disorder, hypertensive encephalopathy, hypoglycemia, and seizures [3].
This patient's blood pressure was continuously monitored and his blood glucose level was immediately tested. Then, brain and vascular images were taken as an early diagnostic method.
For a long time, the treatment of choice for acute ischemic stroke has been intravenous rtPA. Intravenous administration of rtPA is the only FDA-approved medical treatment for patients with AIS. It is associated with improving morbidity and mortality for patients who are treated within 3 hours of stroke onset [3].
There are, however, a significant proportion of patients in whom IV rtPA is contraindicated [9]. Occlusion of proximal arteries such as the proximal internal carotid or middle cerebral arteries has been associated with decreased rates of recanalization after intravenous thrombolysis [9].
IAMT was developed to improve the rates of recanalization by means of endovascular retrieval of larger thrombi. IAMT also provides an alternative revascularization strategy when IV rtPA is contraindicated [10]. Endovascular mechanical thrombectomy can restore vascular patency of vessels between 41 and 54% of the time [11]. IAMT has been shown to increase recanalization rates in AIS when it is used after IV rtPA and when IV rtPA is contraindicated [9]. Several endovascular interventions are being evaluated for the treatment of intracranial or extracranial occlusions leading to AIS [12]. Interventions include mechanical disruption of the clot and extraction of the thrombus. In most cases, the mechanical intervention has been combined with either intra-venous or intra-arterial thrombolytic therapy to patients whose stroke symptom duration is under 8 hours [13].
In the immediate postoperative period, there is a high probability of detection of AIS by the anesthesiologist. There are no guidelines for management of AIS in the perioperative period, but IAMT can be a treatment of choice when thrombolysis is impossible [14].
During the IAMT procedure, conscious sedation or general anesthesia is required according to the preference of the interventional radiologist and the patient condition. General anesthesia is often used to sedate and immobilize the patient to prevent wire-induced vessel injury, facilitate blood pressure control, provide adequate patient ventilation and airway protection, and make the procedure tolerable for patients. Conscious sedation may reduce delays to treatment and allow neurologic assessments during the procedure, which may reveal developing vascular complications. The optimal modality of sedation has not been defined. However, several studies reported that general anesthesia is associated with higher rates of poor neurologic outcome and mortality [15]. There are increasing needs for anesthesiologists during many interventional and diagnostic procedures. Therefore anesthesiologist should understand and investigate the IAMT procedure further.
In conclusion, concerns about postoperative bleeding should not delay the decision making for treatment because immediate revascularization is a critical predictor of AIS prognosis. IAMT can be a treatment of choice for AIS that occurs in the perioperative period, considering the bleeding risk.
References
1. Kam PC, Calcroft RM. Peri-operative stroke in general surgical patients. Anaesthesia. 1997; 52:879–883. PMID: 9349070.

2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011; 114:1289–1296. PMID: 21478735.

3. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007; 38:1655–1711. PMID: 17431204.
4. Ng JL, Chan MT, Gelb AW. Perioperative stroke in noncardiac, nonneurosurgical surgery. Anesthesiology. 2011; 115:879–890. PMID: 21862923.

5. Hariri A, Nourissat G, Dumontier C, Doursounian L. Pulmonary embolism following thrombosis of the brachial vein after shoulder arthroscopy. A case report. Orthop Traumatol Surg Res. 2009; 95:377–379. PMID: 19576863.

6. Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention - cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation - review and meta-analysis. J Intern Med. 2005; 257:399–414. PMID: 15836656.

7. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009; 25:891–896. PMID: 19664509.

8. Schroeder RA, Barbeito A, Bar-Yosef A, Mark JB. Cardiovascular monitoring. In : Miller RD, Eriksson LI, FLeisher LA, Weiner-Kronish JP, Young WL, editors. Miller's anesthesia. 7th ed. Philadelphia: Churchil Livingstone;2010. p. 1267–1328.
9. Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallée PC, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009; 8:802–809. PMID: 19647488.

10. Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004; 35:2848–2854. PMID: 15514171.
11. Berlis A, Lutsep H, Barnwell S, Norbash A, Wechsler L, Jungreis CA, et al. Mechanical thrombolysis in acute ischemic stroke with endovascular photoacoustic recanalization. Stroke. 2004; 35:1112–1116. PMID: 15017011.

12. Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005; 36:2311–2320. PMID: 16179577.
13. Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008; 39:1205–1212. PMID: 18309168.
14. Szeder V, Torbey MT. Prevention and treatment of perioperative stroke. Neurologist. 2008; 14:30–36. PMID: 18195654.

15. John N, Mitchell P, Dowling R, Yan B. Is general anaesthesia preferable to conscious sedation in the treatment of acute ischaemic stroke with intra-arterial mechanical thrombectomy? A review of the literature. Neuroradiology. 2013; 55:93–100. PMID: 22922866.

Fig. 2
Diffusion brain magnetic resonance imaging shows two focal acute infarcts in the left ant frontal lobe and basalganglia, respectively.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download