Abstract
Background
Acinetobacter baumannii (A. baumannii) has emerged globally as a significant pathogen in hospitals. It is also present in soil and water. In a previous study, we discovered that the A. baumannii class 2 integron occurred most frequently. Here, we determined whether the A. baumannii class 2 integron is in the soil around our hospital, and if the soil is the cause for increasing numbers of A. baumannii infections in our intensive care unit (ICU) patients.
Methods
This cross-sectional prospective study was conducted in two ICUs at Loghman-Hakim Hospital, Tehran, Iran, from November 2012 to March 2013. Patient, soil, and hospital environment samples were collected. All isolates were identified using standard bacteriologic and biochemical methods. The phenotypes and genotypes were characterized. The standard disc diffusion method was utilized to test antimicrobial susceptibility. Integron identification was performed by multiplex polymerase chain reaction.
Results
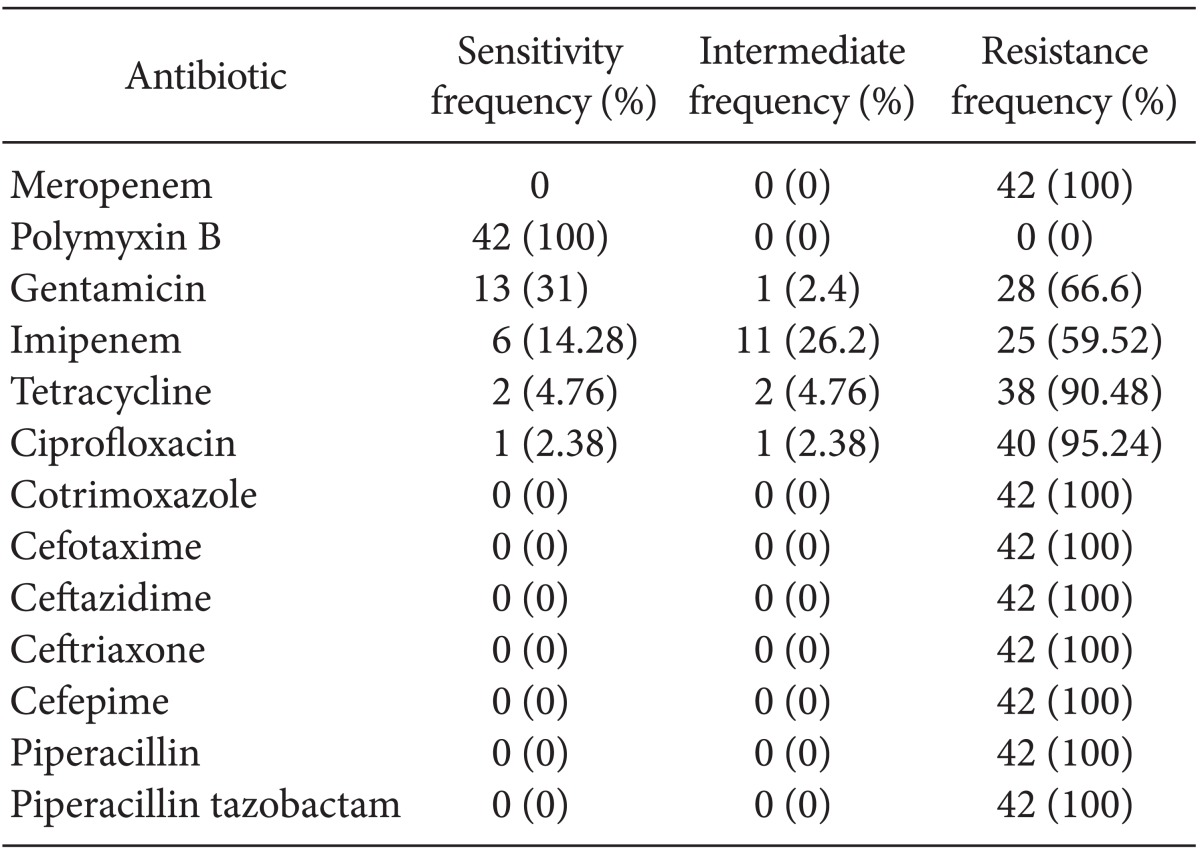
A total of 42 A. baumannii clinical strains were isolated, all from patient samples; 65% of the isolated species were classified as class 2 integrons. The strains were 100% resistant to piperacillin, piperacillin-tazobactam, ceftazidime, ceftriaxone, cotrimoxazole, cefepime, ceropenem, and cefotaxime. However, all of the strains were sensitive to polymyxin B. A. baumannii was detected around the lip of one patient.
Acinetobacter baumannii (A. baumannii) has emerged as a significant pathogen for health care institutes and hospitals globally [1]. This opportunistic human pathogen causes a broad spectrum of nosocomial infections including ventilator-associated pneumonia, meningitis, endocarditis, skin and soft tissue infections, urinary tract infection, conjunctivitis, burn wound infections, and bacteremia, all of which increase the risk of mortality [2,3].
A. baumannii is a non-fermentative, non-motile, oxidase-negative, catalase-positive, Gram-negative bacillus that has developed resistance to several classes of antimicrobial drugs [3]. Similar to other Gram-negative bacteria, particularly psychrophiles, A. baumannii is found in soil and water. A. baumannii isolates that have intermediate sensitivity or are resistant to at least three different classes of antibiotics are known as a multidrug-resistant A. baumannii (MDRAb).
An integron is a mobile capture and gene dissemination system involved in antibiotic-resistant gene dissemination [4]. There are various reports regarding the distribution patterns of the integron classes. The A. baumannii class 1 integron is the most common among MDRAb isolates [4,5]. Class 2 integrons are located on transposon Tn7 [6]. The frequencies of class 1 and 2 integrons are not known. In addition, the association between soil and A. baumannii in Iran has not been established.
In our prior research, we determined that class 2 integrons were the most frequent among Acinetobacter isolates [7]. Also, there has recently been construction in and around our hospital. In the present study, we investigated whether the environment in the intensive care unit (ICU) at Loghman Hakim Hospital, Tehran, Iran, is contaminated with A. baumannii, and if so, which class of integron is most prevalent. We also evaluated the in situ role of inoculums in bioremediation of contaminated soil.
This prospective study was performed from November 2012 to March 2013 in the toxicological ICU (454 patients) and general ICU) (272 patients) units at Loghman Hakim Hospital. The research ethics committee of Shahid Beheshti University, Tehran, Iran, approved the study protocol.
The mean age of patients was 50.47 ± 21.32 years, and 55% of the patients were male. Most (87.18%) were under mechanical ventilation. Sputum was detected in 75% of the cases. All patients with clinical signs including temperature > 38℃, leukocyte count > 10000/µl, development of a purulent respiratory secretion, rales or dullness on chest examination, and A. baumannii outbreaks were defined as clinically suspicious for this microorganism. Patients who developed infections in the wards before admittance to the ICU and patients who received antibiotics < 24 h prior to admission were excluded. Patients who developed infection within 48 h of admission to the ICU were also excluded because any infection they developed most likely was acquired outside the ICU. Inclusion criteria for patients under mechanical ventilation included age > 18 years and at least 48 h on mechanical ventilation with a clinical suspicion of pneumonia. Patients had no clinical evidence of nosocomial infections upon admission to the ICU. A total of 60 patients were determined to be clinically suspicious for Acinetobacter infections. Patient samples included tracheal aspirates for investigation of the lower respiratory tract, urine and blood samples, and wound catheter and nasal swab cultures. Repeatedly isolated species from the same patient were excluded.
All isolates were identified using standard bacteriologic and biochemical methods, which included Gram staining, oxidase tests, oxidation/fermentation (O/F) tests, catalase tests, motility, citrate tests, and growth at 37 and 44℃.
Soil sampling was performed by passing cotton-tipped transfer swabs over the soil within 30 m of the hospital using a standard protocol. Copan Venturi swabs (Copan Diagnostics) were passed over sample surfaces, labeled, and shipped at room temperature to laboratories in two centers including Loghman Hakim and Mofid hospitals. A total of 16 soil samples were evaluated.
Cotton-tipped swabs moistened with BBL (We used either a single culture swab [BBL culturette with liquid Amies; BD Diagnostics] to sample the skin in both the axilla and groin areas, or 2 swabs to sample the axilla and groin areas separately [Scott P 2007]). Trypticase soy broth (BD Diagnostics, Sparks, MD, USA) were placed in the soil samples and streaked across BBL trypticase with 5% sheep blood and BBL MacConkey agar, then incubated at 35℃ for 72 h. Organisms were identified as being a part of the A. baumannii-calcoaceticus complex (ABC) using standard automated biochemical testing methods as described previously [11].
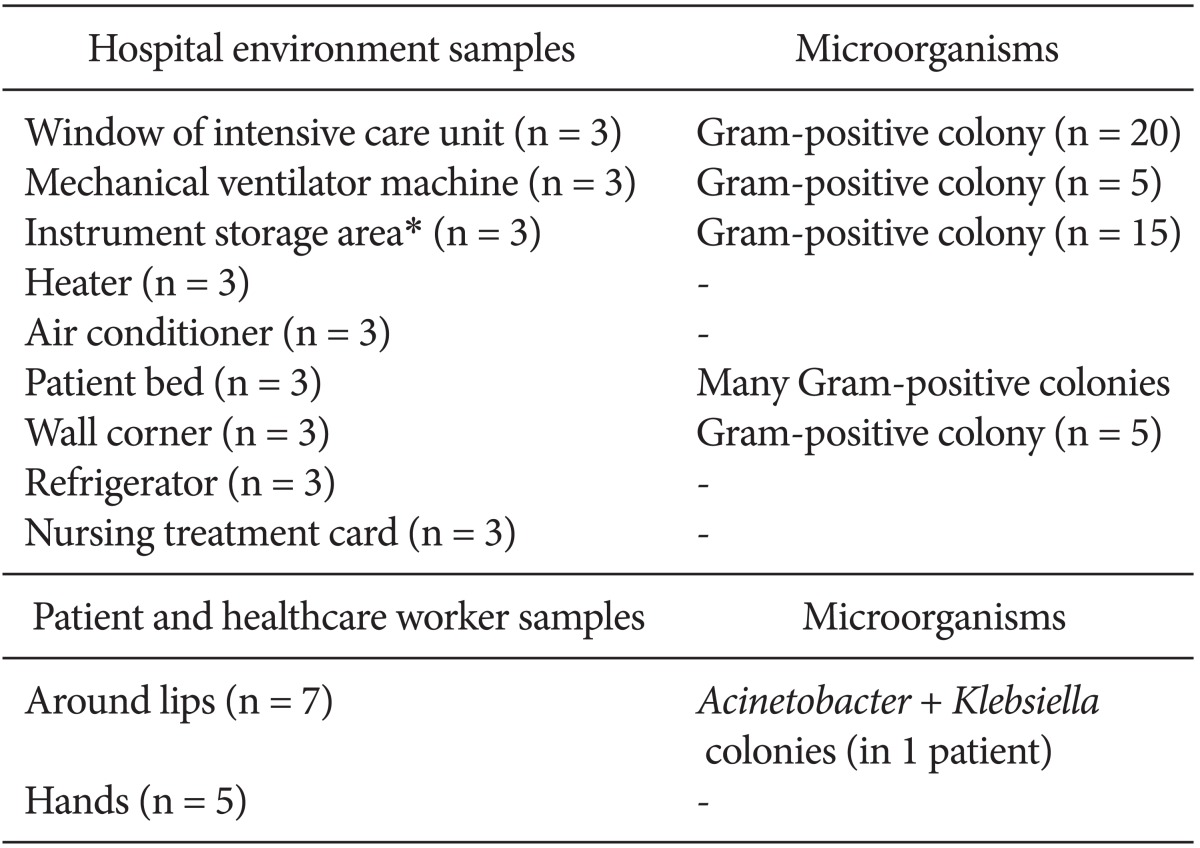
Hospital environment samples were collected from the window of the ICU, equipment such as the mechanical ventilator machine, instrument storage area (The sterilized tools literally on the walls of the ICU), heaters, air conditioners, patient beds, wall corners, refrigerators, and the cardex (nursing treatment cards). Samples from the lips and hands of health care workers and patients were also examined.
Clinical isolates were collected for phenotypic and genotypic characterization.
The standard Mast Co (UK) disc diffusion method was used for antimicrobial susceptibility testing based on the Clinical and Laboratory Standards Institute (CLSI) guidelines for clinical samples. The antibiotics evaluated were as follows: meropenem (10 µg), imipenem (10 µg), gentamicin (10 µg), ceftazidime (10 µg), tetracycline (30 µg), ceftriaxone (30 µg), cefepime (30 µg), cefotaxime (30 µg), ciprofloxacin (5 µg), cotrimoxazole (25 µg), piperacillin (100 µg), piperacillin-tazobactam (100/10 µg), and polymyxin B (300 µg). Pseudomonas aeruginosa ATCC 27853 was used as the control strain [7].
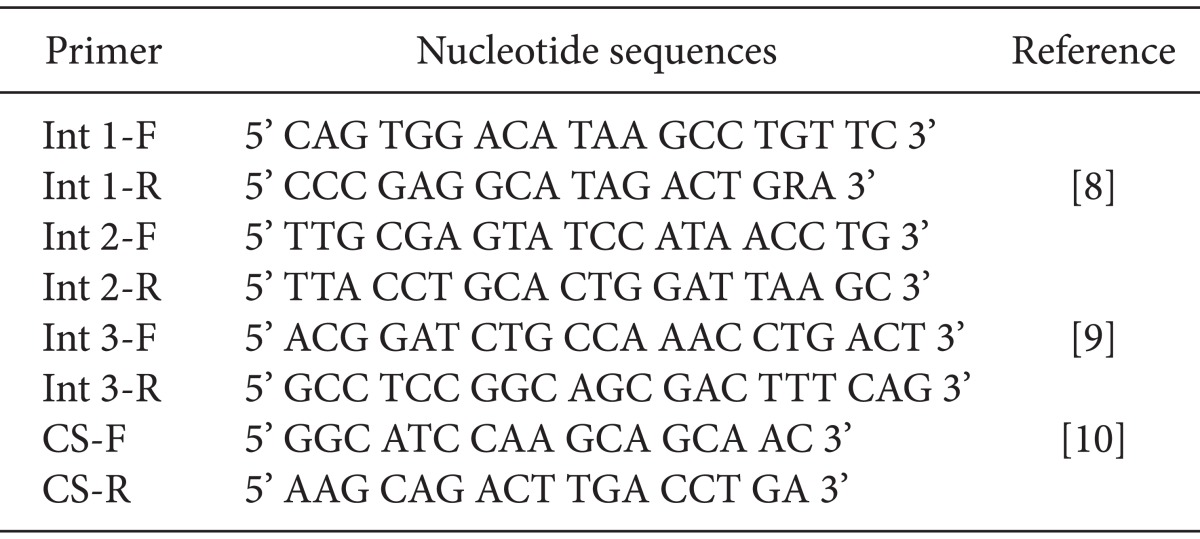
Extraction of genomic DNA from A. baumannii isolates was performed using the iNtRON G-spin™ Total DNA Extraction kit. Determination of integron classes 1 and 2 was performed via multiplex PCR using the primers BioNeer (Table 1) [8,9,10]. PCR of this gene was performed by Int2-R, Int2-F, Int1-R, and Int1-F with the size ranging from 160-288 bp (Table 1). A PCR assay used to define the complete gene makeup of the class 1 integron was performed in 20 µl total volume of (according to the the iNtRON G-spin™ kit)the same concentration mixture previously mentioned.
A total of 42 A. baumannii clinical strains were isolated from the lower respiratory tract (n = 20), blood (n = 6), urine (n = 8), and wound catheters and nasal swabs (n = 8). Of these, 65% (n = 27) of the isolated species were class 2 integrons, 12% (n = 5) were class 1 integrons, and 23% (n = 10) had no integron defined.
Table 2 shows the microorganisms that were isolated. The antibiogram pattern is shown in Table 3. Based on the antibiogram results, all A. baumannii clinical strains were resistant to piperacillin, piperacillin-tazobactam, ceftazidime, ceftriaxone, cotrimoxazole, cefepime, meropenem, and cefotaxime. They showed 4.76, 2.38, 14.28, and 31% sensitivity to tetracycline, ciprofloxacin, imipenem, and gentamicin, respectively, and 100% sensitivity to polymyxin B. There were no A. baumannii bacteria isolated from the hospital environment or soil, although the bacterium was detected around the lip of one patient.
Class 2 integrons are the most common type of A. baumannii isolated among ICU patients at our hospital [7]. This opportunistic human pathogen is often found in soil and water, which is similar to other Gram-negative bacteria [4]. Also, there were 50 buildings under construction around the hospital in the 5 years prior to the study. Given the bioremediation of A. baumannii in soil, we originally believed the outbreaks of A. baumannii in the hospital were related to its presence in the soil. However, the present study indicates otherwise. Prior reports from the ICU during the same months in previous years showed a lower prevalence of A. baumannii than reported in the present study.
In a previous study in Iraq, the majority of infections were caused by antimicrobial-resistant organisms and occurred in soldiers who were critically ill from severe traumatic injuries [11]. Similarly, in the present study, the majority of A. baumannii infections occurred in critically ill patients, particularly in ICU patients with a prolonged duration of hospitalization.
Scott et al. [11] suggested that the source of A. baumannii outbreaks are multifactorial, although the primary source is nosocomial hospital transmission of organisms. Our results are in agreement with that study, although several reports have indicated that environmental exposure is the primary source of infection [12,13,14].
In the present study, the only antimicrobial compound to which A. baumannii was highly susceptible was polymixin B (100% sensitive). It was 100% resistant to meropenem, ceftazidime, ceftriaxone, cefepime, cotrimoxazole, and piperacillin in accordance with a previous study (CLSI 2011). In contrast, Scott et al. [11] found that A. baumannii had >90% susceptibility to imipenem and colistin, and that clinical isolates showed broad-spectrum resistance to fluoroquinolones, cephalosporins, and piperacillin/tazobactam. Considering that we did not isolate A. baumannii from the soil or hospitcal environment, except in one patient with an infection around the lips, other sources of infection should be explored.
In conclusion, further research is necessary to establish a relationship between A. baumannii and soil, (especially in regards to its bioremediation), as well as to determine its importance in nosocomial infections and outbreaks in ICUs.
Acknowledgments
This study is supported by Dr. Pajoumand of Toxicological Research Center of Shahid Beheshti University of Medical Sciences (SBMU).
We would like to express our appreciation to the following colleagues: Dr Rezaei Hemami, Dr. Navidinia, Dr. Valizadeh, Ms. Razi and Ms.Kashi.
References
1. Ramírez MS, Morales A, Vilacoba E, Márquez C, Centrón D. Class 2 integrons dissemination among multidrug resistance (MDR) clones of Acinetobacter baumannii. Curr Microbiol. 2012; 64:290–293. PMID: 22198473.

2. Rit K, Saha R. Multidrug-resistant acinetobacter infection and their susceptibility patterns in a tertiary care hospital. Niger Med J. 2012; 53:126–128. PMID: 23293410.

3. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008; 21:538–582. PMID: 18625687.
4. Taherikalani M, Maleki A, Sadeghifard N, Mohammadzadeh D, Soroush S, Asadollahi P, et al. Dissemination of class 1, 2 and 3 integrons among different multidrug resistant isolates of Acinetobacter baumannii in Tehran hospitals, Iran. Pol J Microbiol. 2011; 60:169–174. PMID: 21905636.

5. Gallego L, Towner KJ. Carriage of class 1 integrons and antibiotic resistance in clinical isolates of Acinetobacter baumannii from northern Spain. J Med Microbiol. 2001; 50:71–77. PMID: 11192508.

6. Japoni S, Japoni A, Farshad S, Ali AA, Jamalidoust M. Association between existence of integrons and multi-drug resistance in Acinetobacter isolated from patients in southern Iran. Pol J Microbiol. 2011; 60:163–168. PMID: 21905635.

7. Kamalbeik S, Kouchek M, Saleh MB, Fallah F, Malekan MA, Talaie H. Prevalence of class 2 integron among multidrug-resistant Acinetobacter baumannii in toxicological ICU patients in Tehran. Iran J Toxicol. 2013; 7:900–906.
8. Koeleman BP, Herr MH, Zavattari P, Dudbridge F, March R, Campbell D, et al. Conditional ETDT analysis of the human leukocyte antigen region in type 1 diabetes. Ann Hum Genet. 2000; 64:215–221. PMID: 11246473.

9. Ploy MC, Denis F, Courvalin P, Lambert T. Molecular characterization of integrons in Acinetobacter baumannii: description of a hybrid class 2 integron. Antimicrob Agents Chemother. 2000; 44:2684–2688. PMID: 10991844.
10. Levesque MA, D'Costa M, Angelopoulou K, Diamandis EP. Time-resolved immunofluorometric assay of p53 protein. Clin Chem. 1995; 41:1720–1729. PMID: 7497612.

11. Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007; 44:1577–1584. PMID: 17516401.

12. Kalenić S, Zele-Starcević L, Jarza-Davila N, Jandrlić M. Infections in war injuries. Lijec Vjesn. 1991; 113:233–235. PMID: 1762486.
13. Oncül O, Keskin O, Acar HV, Küçükardali Y, Evrenkaya R, Atasoyu EM, et al. Hospital-acquired infections following the 1999 Marmara earthquake. J Hosp Infect. 2002; 51:47–51. PMID: 12009820.
14. Maegele M, Gregor S, Steinhausen E, Bouillon B, Heiss MM, Perbix W, et al. The long-distance tertiary air transfer and care of tsunami victims: injury pattern and microbiological and psychological aspects. Crit Care Med. 2005; 33:1136–1140. PMID: 15891349.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download