Abstract
Background
i-gel™ is a new single-use supraglottic airway device without an inflatable cuff. This study was designed to compare the usefulness of i-gel™ versus a classic laryngeal mask airway (cLMA) in small children.
Methods
Sixty-three children (age range : 4-72 months) were randomly assigned to an i-gel™ or cLMA group. We evaluated hemodynamic data, airway sealing ability, the success rate of insertion, and adverse events including an inadvertent sliding out during ventilation.
Results
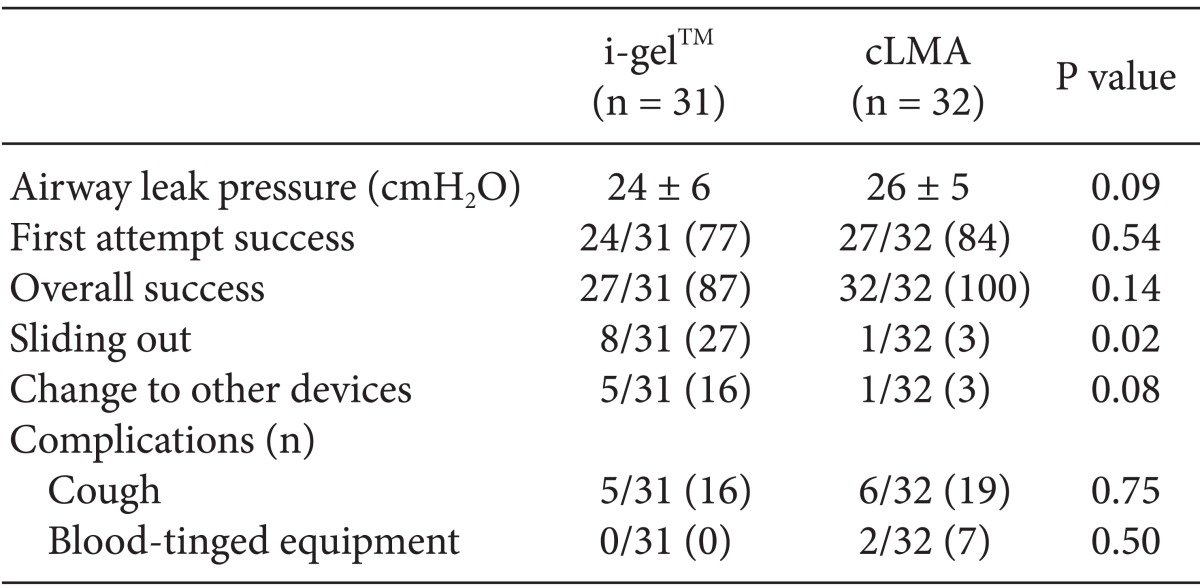
Demographic data and hemodynamic data obtained immediately after the insertion of these devices did not differ between the two groups. The success rates for insertion on the first attempt were 77 and 84% for i-gel™ and cLMA, respectively (P = 0.54), and the overall success rates were 87 and 100% respectively (P = 0.14). There were no significant differences in terms of airway leak pressure. The inserted i-gel™ inadvertently slid out in 8 of 31 patients but only one sliding out case occurred in the cLMA group (P = 0.02). There were no differences between the groups in terms of other side effects (e.g., coughing, bleeding) associated with the use of i-gel™ and cLMA (P = 0.75 and 0.49, respectively).
Conclusions
Oropharyngeal leak pressure and insertion success rate of i-gel™ are similar to those of cLMA. However, i-gel™ is prone to inadvertent sliding out of the mouth in small children. Therefore, it is recommended that the i-gel™ should be secured more tightly to avoid displacement of the device.
Supraglottic airway devices are currently used during pediatric surgeries that require general anesthesia. i-gel™ (Intersurgical Ltd., Wokingham, Berkshire, UK) (Fig. 1) is a recently developed disposable supraglottic airway device. The whole device is made of a soft, gel-like, transparent thermoplastic elastomer (styrene ethylene butadiene styrene) that provides a perilaryngeal seal using a noninflatable cuff [1]. Several studies in pediatric patients have reported that the clinical performance of i-gel™ is comparable to similar devices in terms of insertion time, ease of insertion, and oropharyngeal leak pressure [2-4]. However, i-gel™ is reportedly prone to sliding out of the mouth [5]. Here, we investigated the properties of i-gel™ in comparison with classic laryngeal mask airway (cLMA) in cohort of small children receiving general anesthesia.
After receiving approval from our local ethics committee and parental written informed consent, 63 children (age range: 4-72 months; American society of Anesthesiologists physical status I-II) who were scheduled for polydactyly excision under general anesthesia were included in this study. Exclusion criteria included an increased risk of aspiration, a known difficult airway, congenital malformations involving the respiratory tract, cervical spine disease, and refusal to participate.
Routine monitoring was used through this study, including electrocardiography, heart rate, oxyhemoglobin saturation, noninvasive blood pressure, and end-tidal CO2 (ETCO2). Intravenous propofol (2 mg/kg) was used to induce anesthesia. After the eyelash reflex was lost, bag mask ventilation was provided and rocuronium 0.6 mg/kg was administered. If bag-mask ventilation was adequately maintained using oxygen and sevoflurane, the patient was randomly assigned to receive either i-gel™ or cLMA. All supraglottic airway devices were inserted by two anesthesiologists with considerable experience of LMA insertion. Anesthesia was maintained using 50% nitrous oxide and 2 vol% sevoflurane. Patients were ventilated to a tidal volume of 8-10 ml/kg. The patient's respiratory rate was controlled to maintain the ETCO2 between 30-35 mmHg. An inspiratory-expiratory ratio of 1 : 2 was maintained.
The size of the device was chosen based on the patient's weight (sizes 1.5, 2, and 2.5 for patients weighing between 5-9.9 kg, 10-24.9 kg, and 25-34.9 kg, respectively). Laryngeal masks were lubricated with water-soluble jelly and inserted according to the manufacturer's recommendations. After confirming adequate ventilation, all devices were fixed in place with tape.
We also evaluated hemodynamic data, airway sealing ability (oropharyngeal leak pressure), the success rate of insertion (first attempt and overall success rates), and all adverse events including inadvertent sliding out of the patient. We recorded hemodynamic data before the induction of anesthesia and 1, 3, 5, 7, and 10 minutes after insertion.
Insertion attempts were assessed using following scale: 1, first attempt; 2, second attempt; 3, third attempt; 4, intubation; 5, other method. Successful insertion was confirmed by bilateral chest wall movement, auscultation, and determination of normal capnography curves. A failed insertion was defined as failure to achieve the successful insertion even with minor airway interventions (e.g., adjusting the position of the head and neck, gentle pushing or pulling of the device, holding the device). Device failure was defined as three failed attempts to insert the device. If the third attempt failed, the patient was treated using other methods or intubation. Complications such as coughing, bleeding, and sliding out of the mouth were also recorded.
Oropharyngeal leak pressure was assessed by closing the expiratory valve of the circle system at a fixed gas flow rate of 3 L/min, and the airway pressure was noted (the maximum allowable pressure was 40 cmH2O) [6]. At this time, any gas leaks that were present at the sealing pressure were evaluated by auscultation at the patient's mouth using a stethoscope [7].
Sliding out was defined as a gross emergence of the device from the mouth, or a requirement to use physical force (e.g. adjusting head and neck position, holding the tube) to maintain ventilation despite fixation with tape. If the device was pushed out, we would try to insert it again without removal and then more firmly fix it in place with adhesive bandage. If the ventilation was still not adequate after this corrective procedure, the device would be replaced to other device. During anesthetic maintenance and recovery, side effects (e.g., coughing, bleeding) were recorded by the anesthesiologists.
Previous studies have reported mean leak pressures of 22 and 20 cmH2O for patients treated using the i-gel™ and cLMA, respectively [4]. The standard deviation (SD) of the leak pressure for the i-gel™ group was 6 cmH2O, and a difference of 3 cmH2O was considered clinically relevant. We required more than 30 patients per group to maintain α and β values equal to 0.05 and 0.8, respectively. Data were analyzed to determine normal distributions using the Kolmogorov-Smirnov test. All frequency data were compared with the chi-squared test and continuous data were analyzed with student t-tests or Mann-Whitney tests for the detection of differences between the i-gel™ and cLMA group. Data analysis was performed using Sigmastat 3.1 software. Data are presented as the means ± SD. A P value < 0.05 indicates statistical significance.
The demographic data did not differ between our study groups (n = 31 [I-gel™] vs n = 32 [cLMA]; Table 1). Distribution of the device size in each group was comparable. (Table 2; P = 0.56). There were no differences in terms of the mean blood pressure or heart rates between the two groups. The rates of successful insertion on the first attempt were 77 and 84% using i-gel™ and cLMA, respectively (P = 0.54). The overall success rates of using i-gel™ and cLMA were 87 and 100%, respectively (P = 0.14). The overall success rate with the 1.0 and 1.5 size i-gel™s (79%) was lower than that for the 2.0 and 2.5 size i-gel™ (88%). The leak pressure of the i-gel™ was not significantly different from that of cLMA (24 ± 6 vs. 26 ± 5 cmH2O; P = 0.09). The sliding out incidence was 8 of the 31 total inserted i-gel™s (27%). In five of these cases in which the i-gel™ slid out, the device was subsequently removed and an alternative device was inserted (16%). Only one case of sliding out occurred in the cLMA group, and this patient was subsequently treated using endotracheal intubation. There were no differences in the incidence of other side effects (e.g., coughing, bleeding between the i-gel™ and cLMA groups (Table 3, P = 0.75 and 0.50, respectively).
Our current results indicate that pediatric i-gel™ demonstrates equal performance in terms of leak pressure, success rate, and incidence of adverse events in comparison with cLMA. However, i-gel™ may be prone to inadvertently sliding out of the mouth.
The rate of successful insertion can be used to evaluate the feasibility of using i-gel™. Beylacq et al. [3] reported a successful inserted i-gel™ at the first attempt in all of their pediatric patients (mean age: 12 years). Beringer et al. [2] conducted an i-gel™ study on 120 anesthetized children (mean age: 5 years), and reported first attempt and overall success rates of 92 and 99%, respectively. Theiler et al. [5] compared the use of i-gel™ and the Ambu AuraOnce laryngeal mask device in children (mean age: 6 years), and reported first attempt and overall success rates of 93 and 98%, respectively. Lee et al. [4] also performed a study comparing i-gel™ with cLMA in children (mean age: 3 years), and described first attempt and overall success rates of 96 and 100%, respectively. Our results demonstrate first and overall success rates of 77 and 81%, respectively. These results seem to be very low in comparison with previously published studies. We suppose that the low success rate is due to the younger median age of our current patients and the higher percentage of 1.0-and 1.5-sized devices that were used compared with previous studied. Of particular note, the overall success rate of 1.0- and 1.5-sized i-gel™ devices (79%) was lower than that of 2.0- and 2.5-sized i-gel™ devices (88%).
In our present study, we analyzed oropharyngeal leak pressure to determine airway sealing ability. The median leak pressure of i-gel™ was found to be 24 cmH2O in this study, which is comparable to that reported by Lee et al. [4] of 22 cmH2O. In this study, the leak pressure of i-gel™ was not found to be significantly different from that of cLMA (24 ± 6 vs. 26 ± 5 cmH2O; P = 0.09). Hence, pediatric i-gel™ can provide a safety margin in terms of airway leak pressure for ventilating small children undergoing general anesthesia.
Previous studies have reported that i-gel™ is more prone to sliding out of children in comparison with the Ambu AuraOnce laryngeal mask device [5]. The pronounced airway angle of the Ambu mask provides a better anatomical fit with the laryngeal inlet. Some researchers recommend taping the device in order to prevent intraoperative dislodgment [5]. In our present study, 8 of 31 inserted i-gel™s (27%) became dislodged in comparison with only one inserted cLMAs. Four of these 8 i-gel™s that slid out were less than size 1.5. Five of the 8 i-gel™s that slid out were subsequently removed and replaced with an alternative device. In particular, i-gel™s smaller than size 1.5 required minor interventions after insertion in order to prevent displacement. Because of the straighter and more rigid design of i-gel™ and the relatively anterior and cranial position of the pediatric oropharynx, i-gel™ might demonstrate a tendency to slide out. The reason for such a displacement out of the mouth with the potential to cause partial airway obstruction is unclear but may due to the more conical shape of the hypopharynx in children compared with adults [8]. Once inserted, i-gel™ often needs to be taped in place in order to achieve a sufficient seal and allow ventilation [8]. Consequently, if the patient's position changes, extra vigilance is required to prevent loss of airway pressure.
Adverse events were rare in both of our current study groups. and it was notable that no equipment was stained with blood after device removal. There were also no significant differences found in terms of other side effects (e.g., coughing and bleeding) between i-gel™ and cLMA (P = 0.75 and 0.49, respectively). Although we did not evaluate sore throat after device removal, the non-inflatable cuff of the i-gel™ may diminish the risk of developing sore throat.
One of the limitations of this study was that the data were not collected by observers in the blinded manner. Another limitation is the level of experience of the attending anesthesiologists. The anesthesiologists in this study have much more experience using cLMA than i-gel™. These limitations may have introduced bias to the results.
We conclude from our present analyses that airway sealing ability and insertion success rate of i-gel™ are similar to those of cLMA. However, the overall success rate tends to be lower in small children. Especially, i-gel™ may be prone to sliding out and require a replacement with an alternate airway device in small children.
References
1. Intersurgical. I-gel User Guide. I-gel supraglottic airway, adult and paediatric sizes. Wokingham: Intersurgical;2010.
2. Beringer RM, Kelly F, Cook TM, Nolan J, Hardy R, Simpson T, et al. A cohort evaluation of the paediatric i-gel(™) airway during anaesthesia in 120 children. Anaesthesia. 2011; 66:1121–1126. PMID: 21883132.

3. Beylacq L, Bordes M, Semjen F, Cros AM. The I-gel, a single-use supraglottic airway device with a non-inflatable cuff and an esophageal vent: an observational study in children. Acta Anaesthesiol Scand. 2009; 53:376–379. PMID: 19243322.

4. Lee JR, Kim MS, Kim JT, Byon HJ, Park YH, Kim HS, et al. A randomised trial comparing the i-gel (TM) with the LMA Classic (TM) in children. Anaesthesia. 2012; 67:606–611. PMID: 22352745.
5. Theiler LG, Kleine-Brueggeney M, Luepold B, Stucki F, Seiler S, Urwyler N, et al. Performance of the pediatric-sized i-gel compared with the Ambu AuraOnce laryngeal mask in anesthetized and ventilated children. Anesthesiology. 2011; 115:102–110. PMID: 21572318.

6. Keller C, Brimacombe JR, Keller K, Morris R. Comparison of four methods for assessing airway sealing pressure with the laryngeal mask airway in adult patients. Br J Anaesth. 1999; 82:286–287. PMID: 10365012.

7. Lopez-Gil M, Brimacombe J, Keller C. A comparison of four methods for assessing oropharyngeal leak pressure with the laryngeal mask airway (LMA) in paediatric patients. Paediatr Anaesth. 2001; 11:319–321. PMID: 11359590.
8. Hughes C, Place K, Berg S, Mason D. A clinical evaluation of the I-gel™ supraglottic airway device in children. Paediatr Anaesth. 2012; 22:765–771. PMID: 22672411.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download