Abstract
Cisatracurium was initially characterized to have no evident histamine-releasing potential with excellent cardiovascular stability. However, severe anaphylactic reactions to cisatracurium that resulted in bronchospasms and cardiovascular collapse have been reported worldwide. Two cases of severe anaphylactic reactions after the administration of cisatracurium are presented. The anesthetics used in both cases were lidocaine, midazolam, propofol (microemulsion propofol in the second case), remifentanil and cisatracurium. After the administration of these drugs, bronchospasm and hypotension manifested, leading to the diagnosis of anaphylaxis and appropriate treatment. Skin intradermal testing confirmed that both cases were due to immune-mediated anaphylaxis to cisatracurium, despite the fact that neither of the patients had been exposed to the allergen previously. The anaphylaxis may be due to cross-reactivity between neuromuscular blocking agents and substances with quaternary ammonium ions. Anesthesiologists should be aware that cisatracurium has the potential to trigger severe anaphylactic reactions via an immune-mediated mechanism.
Neuromuscular blocking agents (NMBAs) are the leading cause of perioperative anaphylaxis, with the most common anaphylaxis-inducing agents being succinylcholine, rocuronium and atracurium [1,2]. Cisatracurium, a relatively new NMBA, is a stereoisomer of atracurium, which does not induce apparent histamine release upon a bolus administration and causes fewer allergic reactions than other NMBAs [3]. However, several cases of anaphylactic reactions of varying severity to cisatracurium have been reported [4-7]. Two cases of severe anaphylactic reactions after cisatracurium administration are reported here.
A 52-year-old male (weight, 63 kg; height, 167 cm, American Society of Anesthesiologists physical status classification I) was scheduled for laparoscopic low anterior resection for rectal cancer. He had no significant past medical history and no previous experience of general anesthesia. His family history included his mother's asthma.
The patient arrived at the operating room with no premedication. Cefotetan 1,000 mg IV was administered 20 minutes before arrival with no apparent adverse effects. His blood pressure, heart rate, and SpO2 were 140/80 mmHg, 60 beats/min, and 100%, respectively. After standard monitors were applied, midazolam 2 mg and lidocaine 20 mg were administered. Induction was started with a target effect-site concentration-controlled infusion (TCI; Asan Pump, version 2.0, Bionet Co., Ltd., Seoul, Korea) of propofol 2 µg/ml and remifentanil 3 ng/ml, followed by a bolus administration of cisatracurium 12 mg. Within a minute, mask-valve ventilation became difficult with pulse oximetry decreasing to 90%. His trachea was intubated immediately and ventilated with 100% oxygen. At this point, the peak airway pressure exceeded 30 cmH2O with a tidal volume of 550 ml and a respiratory rate of 12 breaths/min. The breathing sound in both lungs was decreased and wheezing was apparent on auscultation. Signs of cardiovascular collapse were also present: the blood pressure was 60/40 mmHg and there was a slight increase in the heart rate from 65 to 80 beats/min. Cutaneous reactions such as rash or urticaria were absent. A clinical diagnosis of anaphylactic shock was made. The patient was promptly treated with a 500 ml fluid bolus of lactated Ringer's solution and repeated doses of epinephrine10 µg up to a total dose of 50 µg. Arterial and 16 G venous catheters were placed and 500 ml of colloid was loaded. A continuous infusion of epinephrine was started (0.05 µg/kg/min). The blood pressure increased to 125/75 mmHg. Treatment for the bronchospasm was initiated by administering inhaled salbutamol through the endotracheal tube and hydrocortisone 100 mg IV. An hour after the event, the epinephrine infusion was slowly discontinued as the hemodynamics stabilized. The blood pressure and heart rate after epinephrine infusion discontinuation were 135/90 mmHg and 90 beats/min, respectively. The peak airway pressure decreased to 26 cmHg with the same ventilator setting. Wheezing was no longer present on auscultation. The surgery was cancelled and the patient was transported to the surgical intensive care unit with his trachea remaining intubated. One hour after arrival, the patient was awake and extubated without incident. His postoperative chest X-ray was normal.
Three days later, the patient underwent allergy testing with the skin prick test for common allergens (latex included), an intradermal test for all perioperatively used drugs, and the metacholine bronchial provocation test. Other NMBAs such as succinylcholine, vecuronium, and rocuronium were included in the intradermal test drug panel. Skin testing revealed a positive reaction to cisatracurium at a 1 : 100 dilution (Table 1). All other medicines were negative. The patient also reacted positively in the metacholine bronchial provocation test.
A week after the event, the patient was pretreated with methylprednisolone and fluticasone/salmeterol inhaler and returned for the scheduled surgery. Vecuronium was used instead of cisatracurium. The operation was uneventful.
A 70-year-old male (weight, 53.2 kg; height, 155 cm; American Society of Anesthesiologists physical status classification I) was anesthetized for total gastrectomy. The patient had a previous experience with general anesthesia in 1978 for an operation for gastric perforation but information about the anesthetic technique was not available. His past medical history and family history were insignificant.
He received no premedication other than cefotetan 1,000 mg IV, which was administered 20 minutes before his arrival without apparent adverse effects. Full monitoring was established and midazolam 2 mg and lidocaine 40 mg were administered. General anesthesia was induced with TCI (Asan Pump, version 2.0, Bionet Co., Ltd., Seoul, Republic of Korea) of microemulsion propofol 1.2 µg/ml (Aquafol™, Daewon Pharmaceutical Co., Ltd., Seoul, Korea) and remifentanil 3 ng/ml, followed by cisatracurium 12 mg. Approximately 1-2 minutes after induction, the non-invasive blood pressure was unmeasurable and mask-valve ventilation of his lungs was difficult with a SaO2 decrease from 99 to 85%. The patient was intubated immediately and assumed the head-down position. At this point, the measured non-invasive blood pressure was 45/20 mmHg and the heart rate was 70 beats/min. The patient was ventilated with 100% oxygen with a tidal volume of 450 ml and a respiratory rate of 14 breaths/min; the peak airway pressure was 30 cmH2O. On auscultation, wheezing was apparent in both lungs. Repeated doses of epinephrine 20 µg up to a total dose of 40 µg were administered and a continuous infusion of epinephrine was started at 0.1 µg /kg/min. A 16 G catheter was inserted and colloid 500 ml was loaded. An arterial catheter was placed in the right radial artery. The arterial blood pressure increased to 146/90 mmHg and the treatment for bronchospasm with a salbutamol inhaler and hydrocortisone 100 mg IV was started promptly. The hemodynamics stabilized and the peak airway pressure dropped from 30 to 20 cmH2O. The epinephrine infusion was tapered off and eventually discontinued. After monitoring the patient closely for an hour in the operating room, the neuromuscular blockade was reversed with glycopyrrorate 0.4 mg and pyridostigmine 15 mg. The patient was awake and extubated without incident. A follow-up chest X-ray was normal. The patient was transferred to the post anesthesia care unit and had an uneventful recovery.
About 3 weeks later, the patient underwent allergy skin testing, including the skin prick test and the intradermal test for all perioperatively used medicines and other NMBAs. The patient reacted to cisatracurium at a 1 : 100 dilution and showed an equivocal reaction to rocuronium at a 1 : 100 dilution (Table 1). The patient returned for surgery a month after the event and underwent uneventful anesthesia with vecuronium.
Anaphylaxis during general anesthesia is reported to occur with an incidence of 1 : 6,000-1 : 20,000 [1]. The leading causes of perioperative anaphylaxis are the NMBAs, with succinylcholine being the most frequent causative agent, followed by rocuronium and atracurium [1,2]. Cisatracurium, a relatively new non-depolarising NMBA and a stereoisomer of atracurium, was first used clinically in 1995 [3]. Early clinical reports suggested that cisatracurium has negligible histamine-releasing potential and is a less potent trigger of allergic reactions than other NMBAs [3]. However, since 1995, several cases of cisatracurium-induced anaphylactic reactions with severe symptoms of cardiovascular collapse and bronchospasm have been reported [4-7].
Anaphylaxis is a severe, life-threatening, systemic hypersensitivity reaction. It is caused by the degranulation of mast cells or basophiles that results in the release of preformed mediators, including histamine and tryptase. These mediators can affect one or more organ systems such as the skin and the cardiovascular, respiratory, and gastrointestinal systems. The mechanisms that cause mast cell degranulation can be divided into immunemediated (IgE-mediated, anaphylactic) reactions and nonimmune-mediated (chemically-mediated, anaphylactoid) reactions [8]. The two types are indistinguishable clinically but the immune-mediated type tends to cause more severe reactions (cardiovascular collapse and bronchospasm) [9]. A diagnosis of immune-mediated anaphylaxis is often confirmed by biochemical studies such as those measuring serum tryptase levels. Tryptase is one of the mediators that are released by activated mast cells in immune-mediated anaphylactic reactions. Unlike histamine, which peaks immediately after the reaction and then decreases with a half-life of 20 minutes, the half-life of tryptase is 90 minutes and can be detected for 6 or more hours after anaphylaxis onset [10]. Unfortunately, the tryptase test was not used in our cases. However, both cases were positive for the intradermal skin test for cisatracurium, which is highly indicative of immune-mediated anaphylaxis. Therefore, the term 'anaphylactic reaction' used in this report refers specifically to immune-mediated anaphylaxis. To confirm that the patients had an immune-mediated anaphylactic reaction, it would be necessary to perform allergen-specific IgE antibody tests (e.g., the radioallergosorbent test). However, these tests are not currently available for cisatracurium [1,5].
Immune-mediated anaphylaxis to NMBAs does not necessarily require prior exposure to the causative agent. As in our cases, many patients with severe anaphylactic reactions were not exposed previously to any NMBA [4-7]. The anaphylaxis may arise due to cross-reactivity to the tertiary and quaternary ammonium ions that are often found in foods, drugs and cosmetics [11]. Cross-reactivity to the NMBAs is also common [9]. Therefore, for patients who have experienced anaphylaxis to one NMBA, it is essential that the allergic skin test panel contain all available NMBAs. We included succinylcholine, vecuronium, rocuronium, and cisatracurium in the skin tests. The second patient not only reacted to cisatracurium, he also reacted equivocally to rocuronium, which suggests that this patient may cross-react to both NMBAs (Table 1).
Regardless of the underlying mechanism, anaphylaxis treatment should aim to maintain airway patency, intravascular fluid volume, vascular tone, and cardiac output [1]. The administration of possible causative drug(s) should be stopped immediately and 100% oxygen should be given while securing airway patency. The breathing sounds and peripheral pulses should be checked and if these are absent, cardiopulmonary resuscitation must be instituted immediately. The intravascular volume should be maintained with crystalloid and colloid (colloid should be avoided if it was given prior to the event) [1]. Epinephrine in aliquots of 5-500 µg, depending on the severity of anaphylaxis, should be given repeatedly until the patient responds [1,12]. Continuous infusion of epinephrine may be required if the patient remains hypotensive. Bronchospasms should be treated with b2-agonists and as the patient stabilizes hemodynamically, an H1-blocker and hydrocortisone should be given intravenously [13]. It is recommended that the surgery be delayed. However, if the surgery is emergent and must be continued, any hemodynamic instability should be managed judiciously. Fibrinolysis during anaphylaxis has been reported and should be kept in mind as it could increase the risk of perioperative bleeding [14].
Two cases of severe anaphylactic reactions to cisatracurium are presented here. Both cases were confirmed by positive skin test results to be immune-mediated anaphylaxis. While cisatracurium may have a low histamine-releasing potential and therefore rarely causes chemically-mediated anaphylactoid reactions, our cases and several other cases indicate that it has the potential to induce immune-mediated anaphylaxis, regardless of prior exposure.
References
1. Nel L, Eren E. Peri-operative anaphylaxis. Br J Clin Pharmacol. 2011; 71:647–658. PMID: 21235622.

2. Mertes PM, Alla F, Tréchot P, Auroy Y, Jougla E. Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011; 128:366–373. PMID: 21497888.
3. Wastila WB, Maehr RB, Turner GL, Hill DA, Savarese JJ. Comparative pharmacology of cisatracurium (51W89), atracurium, and five isomers in cats. Anesthesiology. 1996; 85:169–177. PMID: 8694363.

4. Krombach J, Hunzelmann N, Kőster F, Bischoff A, Hoffmann-Menzel H, Buzello W. Anaphylactoid reactions after cisatracurium administration in six patients. Anesth Analg. 2001; 93:1257–1259. PMID: 11682408.

5. Toh KW, Deacock SJ, Fawcett WJ. Severe anaphylactic reaction to cisatracurium. Anesth Analg. 1999; 88:462–464. PMID: 9972775.

6. Clendenen SR, Harper JV, Wharen RE Jr, Guarderas JC. Anaphylactic reaction after cisatracurium. Anesthesiology. 1997; 87:690–692. PMID: 9316977.

7. Briassoulis G, Hatzis T, Mammi P, Alikatora A. Persistent anaphylactic reaction after induction with thiopentone and cisatracurium. Paediatr Anaesth. 2000; 10:429–434. PMID: 10886702.

8. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004; 113:832–836. PMID: 15131563.

9. Mertes PM, Aimone-Gastin I, Guéant-Rodriguez RM, Mouton-Faivre C, Audibert G, O'Brien J, et al. Hypersensitivity reactions to neuromuscular blocking agents. Curr Pharm Des. 2008; 14:2809–2825. PMID: 18991700.

10. Ebo DG, Fisher MM, Hagendorens MM, Bridts CH, Stevens WJ. Anaphylaxis during anaesthesia: diagnostic approach. Allergy. 2007; 62:471–487. PMID: 17441788.

11. Baldo BA, Fisher MM. Substituted ammonium ions as allergenic determinants in drug allergy. Nature. 1983; 306:262–264. PMID: 6196640.

12. Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003; 97:1381–1395. PMID: 14570656.

13. Harper NJ, Dixon T, Dugué P, Edgar DM, Fay A, Gooi HC, et al. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009; 64:199–211. PMID: 19143700.
14. De Souza RL, Short T, Warman GR, Maclennan N, Young Y. Anaphylaxis with associated fibrinolysis, reversed with tranexamic acid and demonstrated by thrombelastography. Anaesth Intensive Care. 2004; 32:580–587. PMID: 15675222.

15. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002; 57:45–51. PMID: 11991289.

Table 1
Results of the Skin Intradermal Test
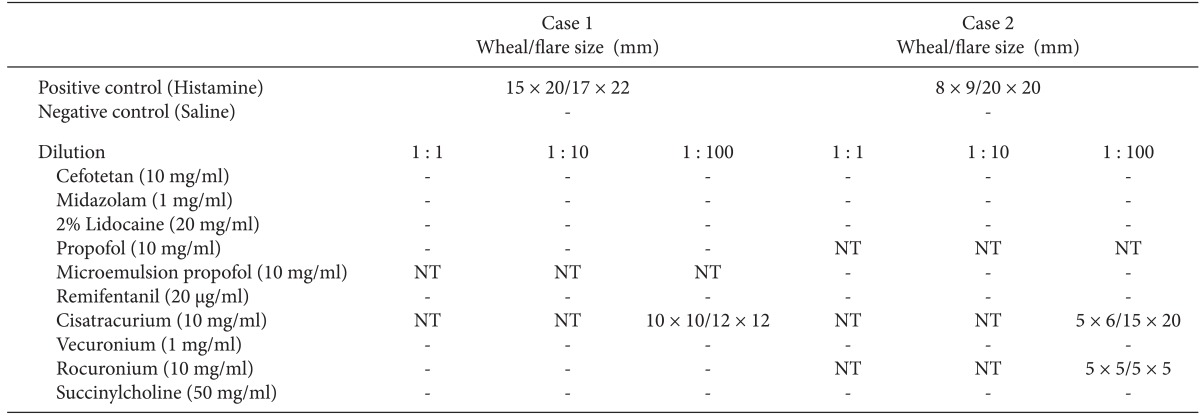
Intradermal tests were performed by injecting 0.1 ml into the dermis of the forearm or back via a hypodermic needle. The reactions were read after 20 min. A result was considered to be positive if the diameter of the initial wheal increased in size by 3 mm or greater after 15-20 min and was associated with a flare [15]. The serial dilutions started with a 1/100 dilution. The injection dilutions increased progressively as long as the results remained negative [10]. NT: not tested.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download