Abstract
Background
Postoperative nausea and vomiting (PONV) continues to be a major problem, because PONV is associated with delayed recovery and prolonged hospital stay. Although the PONV guidelines recommended the use of 5-hydroxy-tryptamine (5-HT3) receptor antagonists as the first-line prophylactic agents in patients categorized as high-risk, there are few studies comparing the efficacies of ondansetron, ramosetron, and palonosetron. The aim of present study was to compare the prophylactic antiemetic efficacies of three 5HT3 receptor antagonists in high-risk patients after laparoscopic surgery.
Methods
In this prospective, randomized, double-blinded trial, 109 female nonsmokers scheduled for elective laparoscopic surgery were randomized to receive intravenous 4 mg ondansetron (n = 35), 0.3 mg ramosetron (n = 38), or 75 µg palonosetron (n = 36) before anesthesia. Fentanyl-based intravenous patient-controlled analgesia was administered for 48 h after surgery. Primary antiemetic efficacy variables were the incidence and severity of nausea, the frequency of emetic episodes during the first 48 h after surgery, and the need to use a rescue antiemetic medication.
Results
The overall incidence of nausea/retching/vomiting was lower in the palonosetron (22.2%/11.1%/5.6%) than in the ondansetron (77.1%/48.6%/28.6%) and ramosetron (60.5%/28.9%/18.4%) groups. The rescue antiemetic therapy was required less frequently in the palonosetron group than the other groups (P < 0.001). Kaplan-Meier analysis showed that the order of prophylactic efficacy in delaying the interval to use of a rescue emetic was palonosetron, ramosetron, and ondansetron.
Postoperative nausea and vomiting (PONV) continues to be a major problem although avoiding PONV is a high priority for patients and physicians. Apart from patient discomfort, PONV is associated with adverse effects, such as delayed recovery and prolonged hospital stay. Although rare, postoperative morbidities including wound dehiscence, pulmonary aspiration, bleeding, and dehydration that can occur if vomiting is prolonged [1]. Established patient-specific risk factors for PONV include female gender, nonsmoking, and a history of motion sickness or PONV, whereas nonspecific factors are postoperative opioids use and type of surgery such as laparoscopy [2-4].
Ondansetron was the first commercially available 5-hydroxytryptamine (5-HT3) receptor antagonist, thereafter granisetron, dolasetron, tropisetron, ramosetron, and palonosetron were introduced. Many studies have confirmed that this class of antiemetics exhibited better prophylactic efficacies compared with the older traditional drugs including droperidol, perphenazine, or metoclopramide [5-7]. A number of studies have been conducted to compare the prophylactic antiemetic efficacies among 5-HT3 receptor antagonists [8-12]. To date, however, there are few clinical studies comparing the prophylactic efficacies of ondansetron, ramosetron, and palonosetron in high-risk patients with PONV.
The aim of this prospective, randomized, double-blinded trial was to compare the prophylactic antiemetic efficacies of ondansetron, ramosetron, and palonosetron in high-risk patients with fentanyl-based PCA after laparoscopic surgery. Our hypothesis was that three 5HT3 receptor antagonists show different antiemetic efficacies when used prophylactically.
Our study was conducted from July 2010 to June 2011 and 109 patients were enrolled. The inclusion criteria were as follows: 1) American Society of Anesthesiologists physical status I-II, 2) female patient aged 20-65 years, 3) nonsmoker, 4) history of motion sickness or previous PONV, 5) elective laparoscopic surgery, and 6) use of postoperative intravenous patient-controlled analgesia (IV-PCA) using fentanyl. The exclusion criteria were: impairment of bowel motility, diabetes, pregnancy or lactation, administration of an antiemetic medication or steroids within 24 h before surgery, the presence of a cardiovascular or respiratory disease, obesity (body mass index > 35 kg/m2), or renal or hepatic dysfunction. The plan of the study was reviewed and approved by the Institutional Review Board of Yonsei University Health Systems) and written informed consent was obtained from all patients.
Using computer-generated random numbers, the hospital pharmacy that prepared the study drugs assigned patients into one of 3 active treatment groups on the morning of the day of surgery. Of the 109 patients, 35 were randomized to receive 4 mg ondansetron (ondansetron group), 38 mg to 0.3 mg ramosetron (ramosetron group), or 36 µg to 75 µg palonosetron (palonosetron group). Each study drug was mixed with saline to a total volume of 3 ml in an unlabeled syringe and was intravenously administered just prior to induction of anesthesia. All patients, surgeons, anesthesiologists, and nurses involved in the study were blinded to group allocation and every precaution was taken to maintain the double-blind conditions.
No patient received premedication. Intraoperative monitoring included electrocardiography, blood pressure measurement, peripheral oxygen saturation (SpO2), and end-tidal CO2 tension (ETco2). General anesthesia was induced using 2 mg/kg of propofol and 1 µg/kg of remifentanil infusion. After tracheal intubation using 0.6 mg/kg of rocuronium, anesthesia was maintained with sevoflurane in 50% oxygen/air. Sevoflurane concentration was adjusted to ensure an equal depth of anesthesia during surgery as assessed by the bispectral index (BIS; BIS A-1050 Monitor, Aspect Medical Systems, Newton, MA, USA), which was held between 40-60. Remifentanil was administered for supplemental intraoperative analgesia and its dose was adjusted to maintain blood pressure and a heart rate within 20% of baseline values. Patients emerging from anesthesia were managed in the postanesthetic care unit and in the ward by an anesthesiologist blinded to group allocation. All intraoperative variables including total amounts of infused remifentanil, administered fluid, urine output, and estimated blood loss were also counted by blinded anesthesiologist.
IV-PCA devices (Ambix anaplus, E-Wha Fresenius Kabi Inc., Gunpo, Republic of Korea) with 0.2 µg/kg/ml of fentanyl were commenced at the end of surgery with each device programmed to deliver 1 ml/h as a background infusion and 1 ml per demand with a 15 min lockout time over a 48 h period.
Primary antiemetic efficacy variables were the incidence and severity of nausea, the frequency of emetic episodes during the first 48 h after surgery, and the need to use a rescue antiemetic medication. Nausea was defined as a subjective desire to vomit, but without expulsive muscular movements. Nausea was scored on an 11-point verbal rating scale from 0 (no nausea) to 10 (worst possible nausea): severity was scored as mild (1-3), moderate (4-6), or severe (7-10) [13]. Retching was defined as an expulsive movement of the stomach muscles without expulsion of stomach contents [14]; both retching and vomiting were considered to be emetic episodes [14,15].
If patients complained of moderate to severe nausea, had any emetic episode, or requested a rescue drug, 20 mg of propofol was administered repeatedly for treatment in the PACU. When rescue antiemetics were needed in the ward, 10 mg of metoclopramide was given. If PONV occurred more than 6 h after surgery, 4 mg of ondansetron or/and 4 mg of dexamethasone was/were administered at the discretion of attending anesthesiologists. Dexamethasone administration was not repeated more than once for 8 h. The interval to the first administration of a rescue antiemetic commenced at the end of surgery. Postoperative nausea and emetic episodes were assessed for 48 h after surgery by 2 independent investigators blinded to patient group. Patients were assessed over 5 time periods: 0-1 h, 1-6 h, 6-24 h, and 24-48 h after surgery. IVPCA was discontinued when severe nausea persisted despite treatment with rescue antiemetics and/or at the patient's request. Patients complaining of pain after discontinuation of IV-PCA were given 30 mg of intravenous ketorolac.
Secondary efficacy variables included postoperative pain intensity and the total amount of fentanyl administered via IV-PCA. Pain intensity was measured using an 11-point VRS ranging from 0 (no pain) to 10 (worst pain imaginable) at 1 h, 6 h, 24 h, and 48 h after surgery.
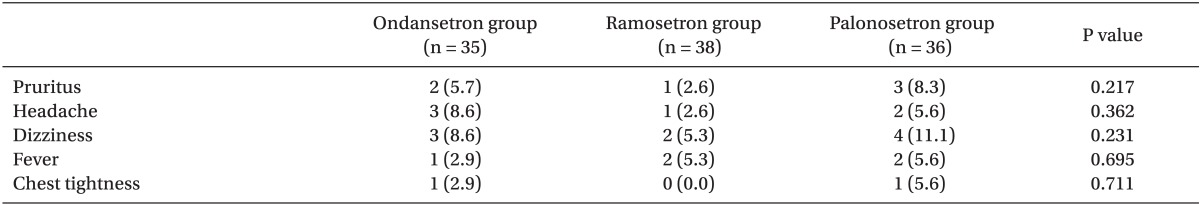
The most frequently reported side-effects of 5-HT3 including headache, dizziness, and drowsiness were also assessed during the study period [14].
Sample size was calculated with reference to the results of a study comparing the effects of ramosetron and ondansetron on PONV associated with the use of IV-PCA in highly susceptible patients [9]. We calculated that the inclusion of 35 patients per group would afford an 80% chance of detection of a 20% reduction in the incidence of PONV using the Fisher's exact test with a type I error of 0.05. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to ensure that data were normally distributed. Patient characteristics and intra- and post-operative variables were analyzed by two-tailed ANOVA. Intergroup differences in nonparametric variables were compared using the Kruskal-Wallis test followed by Dunn's multiple comparisons. Categorical data were compared using the chi-squared test or the Fisher's exact test. The Kaplan-Meier method was used to determine the intervals to the first use of rescue antiemetic in the 3 groups; the curves were compared using the log rank test (Mantel-Cox). A P value < 0.05 was considered statistically significant.
No patient withdrew from the study. Neither patient characteristics nor intraoperative data differed among the 3 groups (Table 1).
The number of nausea-free patients was greater in the palonosetron group than in the other 2 groups during the 48 h study period (Table 2). The number of nausea-free patients was also greater in the ramosetron group than in the ondansetron group overall. During the first hour after surgery, the overall number of patients who experienced postoperative nausea was low and not significantly different among the groups. The rescue antiemetic was used less frequently in the palonosetron group than in the other 2 groups. Patients who were given the rescue antiemetic were censored and Kaplan-Meier analysis of the interval to first rescue antiemetic showed a significant intergroup difference (Fig. 1) between the palonosetron and ondansetron (P < 0.001), palonosetron and ramosetron (P < 0.001), and ondansetron and ramosetron (P = 0.042) groups. Thus, the order of prophylactic efficacy in prolonging the time to use a rescue emetic was palonosetron, ramosetron, and ondansetron.
Six patients (17.1%) in the ondansetron group, 5 (14.3%) in the ramosetron group, and none (0%) in the palonosetron group (P = 0.041) requested disconnection of the IV-PCA pumps because of intractable nausea and/or vomiting after surgery mostly within 12 h.
This prospective, randomized, double-blinded trial compared the prophylactic antiemetic efficacies of ondansetron, ramosetron, and palonosetron in high-risk patients. Palonosetron was superior to ondansetron or ramosetron when used prophylactically to reduce the incidence and severity of PONV and to delay the use of rescue antiemetics.
Consistent with previous findings [9], we observed very high incidences of PONV (80%) despite the prophylactic use of ondansetron. This is mainly due to the patient's factor. All study patients were at high-risk because they had at least 4 predictors of PONV: female gender, nonsmoker, use of postoperative opioids, and history of motion sickness or previous PONV. The incidence of PONV increases exponentially from 10% when no risk factor is present to 79% when 4 risk factors are all present [4,14]. Laparoscopic surgery is also highly susceptible to PONV because abdominal gas insufflation may stretch mechanoreceptors of the intestine and consequently activate 5-HT3 receptors via serotonin release [16]. Another explanation for the frequent PONV in our results is the use of fentanyl-based IV-PCA, especially the use of basal infusion with relatively low demand capacity of fentanyl in our settings. Adding a basal infusion to IV-PCA could be more convenient in surgery and more effective in reducing resting pain than bolus demand only, but it may be associated with a greater risk of PONV. In addition, other reasons for unsatisfactory antiemetic effects of ondansetron given prophylactically are the timing of administration [17], the dose of ondansetrone [18], and CYP2D6 alleles polymorphisms [19].
Although ondansetron has been used to prevent PONV [20,21], there are conflicting results about its effect on preventing IV-PCA related PONV, which were not clinically satisfactory [22,23]. In our results, there was no difference among the groups in terms of the number of patients who experienced nausea and emetic episodes in the first hour after surgery. However, after that period, ondansetron did not reduce fentanyl-based IV-PCA related nausea and emetic episodes. Ramosetron, a newer 5-HT3 antagonist, has been reported to be an effective antiemetic in patients undergoing various types of surgery. The elimination half-life of ramosetron (5.8 ± 1.2 h) is longer than that of ondansetron (3.8 ± 1.0 h) [24]. Two recent, randomized studies compared the prophylactic antiemetic efficacies of ondansetron and ramosetron in patients receiving opioid-based PCA [9,10] The former of the 2 studies found that the incidence of nausea was similar in the 2 groups, but that ramosetron decreased the severity of nausea and the incidence of vomiting, resulting in a reduced need for antiemetic rescue treatment [9]. The latter of the 2 studies showed that the rate of PONV and the use of rescue antiemetics were lower in the patients who received ramosetron than in patients who received ondansetron [10]. The present study showed that the ramosetron group had more patients who presented nausea-free postoperatively compared with the ondansetron group. During first 1-6 h after surgery, fewer patients who had emetic episodes were also in the ramosetron group than in the ondansetron group, despite the similar severity of nausea. In addition, Kaplan-Meier analysis revealed that ramosetron significantly prolonged the time to use a rescue emetic compared with ondansetron, which is attributable to failure of prophylaxis.
Palonosetron, the latest 5-HT3 receptor antagonist introduced in 2003, has been proven to be effective when used to prevent emesis associated with chemotherapy [25]. The minimum effective dose of palonosetron was 75 µg and the drug has been approved by the United States Food and Drug Administration (FDA) for PONV prophylaxis [26,27]. We found that palonosetron exerted a greater prophylactic effect than did either ondansetron or ramosetron. Kaplan-Meier analysis also showed that the efficacy of palonosetron was superior to that of the other 2 drugs. The greater efficacy of palonosetron may be attributable to the fact that the binding affinity to the 5-HT3 receptor is 30 times higher than that of either ondansetron or ramosetron and/or to the extended half-life of palonosetron (approximately 40 h, thus 4-10-fold longer than that of the older antagonists) [28]. However, these properties do not entirely explain the higher efficacy of palonosetron relative to ondansetron or ramosetron. If efficacy was attributable to potency alone, ondansetron or ramosetron could be administered at higher doses. If half-life was all-important, other drugs with shorter half-lives could be injected more often. However, administration of ondansetron for more than 24 h after chemotherapy did not prolong the emesis-free interval afforded by the use of palonosetron [29], suggesting that the longer half-life of palonosetron does not explain the greater antiemetic efficacy of the drug when given within 24 h after surgery. Palonosetron may be unique in terms of allosteric interaction with and binding cooperativity to 5-HT3 receptors [30]. Moreover, binding of palonosetron to the receptors and thereby inhibiting calcium influx is not easily reversible, suggesting that palonosetron uniquely triggers 5-HT3 receptor internalization and induces prolonged inhibition of receptor function [30].
Our study has several limitations. First, we did not include a placebo control group to evaluate the baseline incidence of PONV as we considered it unethical to withhold prophylactic antiemetic drugs in patients at high risk for PONV. Second, patient satisfaction was not an end-point. Third, combination therapy has been shown to be more effective to treat or prevent PONV, especially in high-risk patients. For example, corticosteroids such as dexamethasone are frequently combined with agents of other drug classes, such as metoclopramide or 5-HT3 antagonists, resulting in significant improvements in response. Future research should assess the use of drug combinations to prevent PONV in high-risk patients; the work should also evaluate drug cost-effectiveness. It is also necessary to assess the efficacy of antiemetic drugs in the treatment of established PONV.
In conclusion, a single intravenous injection of 75 µg of palonosetron was more effective in preventing PONV and reduced the need to use a rescue antiemetic compared to an injection of 4 mg of ondansetron or 0.3 mg of ramosetron in high-risk patients who were scheduled for laparoscopic surgery with fentanyl-based postoperative pain management.
References
1. Habib AS, Gan TJ. Pharmacotherapy of postoperative nausea and vomiting. Expert Opin Pharmacother. 2003; 4:457–473. PMID: 12667109.

2. Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003; 97:62–71. PMID: 12818945.

3. Eberhart LH, Hogel J, Seeling W, Staack AM, Geldner G, Georgieff M. Evaluation of three risk scores to predict postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2000; 44:480–488. PMID: 10757586.

4. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999; 91:693–700. PMID: 10485781.
5. Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000; 59:213–243. PMID: 10730546.

6. Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011; 2011:748031. PMID: 22110499.

7. Alon E, Himmelseher S. Ondansetron in the treatment of postoperative vomiting: a randomized, double-blind comparison with droperidol and metoclopramide. Anesth Analg. 1992; 75:561–565. PMID: 1388337.
8. Naguib M, el Bakry AK, Khoshim MH, Channa AB, el Gammal M, el Gammal K, et al. Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron and metoclopramide in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind comparison with placebo. Can J Anaesth. 1996; 43:226–231. PMID: 8829860.

9. Choi YS, Shim JK, Yoon do H, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976). 2008; 33:E602–E606. PMID: 18670328.
10. Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010; 65:500–504. PMID: 20337618.

11. Choi YS, Shim JK, Ahn SH, Kwak YL. Efficacy comparison of ramosetron with ondansetron on preventing nausea and vomiting in high-risk patients following spine surgery with a single bolus of dexamethasone as an adjunct. Korean J Anesthesiol. 2012; 62:543–547. PMID: 22778890.

12. Lee JW, Park HJ, Choi J, Park SJ, Kang H, Kim EG. Comparison of ramosetron's and ondansetron's preventive anti-emetic effects in highly susceptible patients undergoing abdominal hysterectomy. Korean J Anesthesiol. 2011; 61:488–492. PMID: 22220226.

13. Boogaerts JG, Vanacker E, Seidel L, Albert A, Bardiau FM. Assessment of postoperative nausea using a visual analogue scale. Acta Anaesthesiol Scand. 2000; 44:470–474. PMID: 10757584.

14. Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002; 46:921–928. PMID: 12190791.
15. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004; 350:2441–2451. PMID: 15190136.

16. Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006; 102:1884–1898. PMID: 16717343.

17. Tang J, Wang B, White PF, Watcha MF, Qi J, Wender RH. The effect of timing of ondansetron administration on its efficacy, cost-effectiveness, and cost-benefit as a prophylactic antiemetic in the ambulatory setting. Anesth Analg. 1998; 86:274–282. PMID: 9459232.

18. Figueredo E, Canosa L. Prophylactic ondansetron for postoperative emesis. Meta-analysis of its effectiveness in patients with previous history of postoperative nausea and vomiting. Acta Anaesthesiol Scand. 1999; 43:637–644. PMID: 10408818.
19. Candiotti KA, Birnbach DJ, Lubarsky DA, Nhuch F, Kamat A, Koch WH, et al. The impact of pharmacogenomics on postoperative nausea and vomiting: do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology. 2005; 102:543–549. PMID: 15731591.
20. Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ. Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997; 87:1277–1289. PMID: 9416710.
21. White PF, Watcha MF. Postoperative nausea and vomiting: prophylaxis versus treatment. Anesth Analg. 1999; 89:1337–1339. PMID: 10589604.

22. Dresner M, Dean S, Lumb A, Bellamy M. High-dose ondansetron regimen vs droperidol for morphine patient-controlled analgesia. Br J Anaesth. 1998; 81:384–386. PMID: 9861125.

23. Millo J, Siddons M, Innes R, Laurie PS. Randomised double-blind comparison of ondansetron and droperidol to prevent postoperative nausea and vomiting associated with patient-controlled analgesia. Anaesthesia. 2001; 56:60–65. PMID: 11167438.

24. Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc). 2002; 38:75–89. PMID: 12532186.

25. Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003; 98:2473–2482. PMID: 14635083.
26. Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008; 107:439–444. PMID: 18633021.

27. Candiotti KA, Kovac AL, Melson TI, Clerici G, Joo Gan T. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomiting. Anesth Analg. 2008; 107:445–451. PMID: 18633022.

28. Aapro MS. Palonosetron as an anti-emetic and anti-nausea agent in oncology. Ther Clin Risk Manag. 2007; 3:1009–1020. PMID: 18516316.
29. Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005; 23:1289–1294. PMID: 15718327.

30. Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010; 626:193–199. PMID: 19836386.

Fig. 1
Kaplan-Meier analysis of the intervals before administering the first dose of rescue antiemetic in the palonosetron, ondansetron, and ramosetron groups. Significant differences were observed between the palonosetron and ondansetron (P < 0.001), palonosetron and ramosetron (P < 0.001), and ondansetron and ramosetron (P = 0.042) groups.
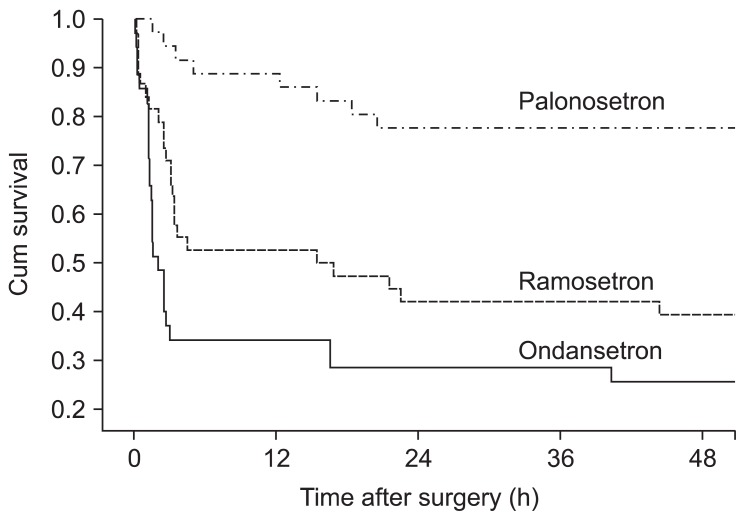
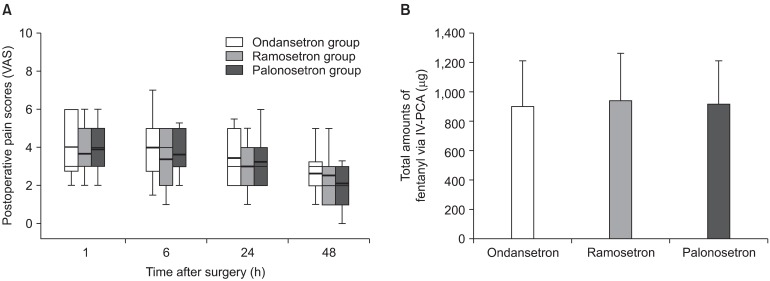
Fig. 2
(A) Postoperative pain scores (VAS) at 1 h, 6 h, 24 h, and 48 h after surgery in the palonosetron, ondansetron, and ramosetron groups. Data are shown as box plots with ranges (whiskers), interquartile ranges (boxes), medians (solid lines), and means (bold lines). There was no difference in VAS pain score among the groups during the study period. (B) Total amounts of fentanyl administered via intravenous patient-controlled analgesia (IV-PCA) in the 3 groups during 48 h after surgery. Data are shown as mean with SD.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download