This article has been
cited by other articles in ScienceCentral.
Abstract
Background
5-HT3 receptor antagonist, dexamethasone and droperidol were used for the prevention of postoperative nausea and vomiting (PONV). Recently, neurokinin-1 (NK1) antagonist has been used for PONV. We evaluated the effect of oral aprepitant premedication in addition to ondansetron.
Methods
A total 90 patients scheduled for elective rhinolaryngological surgery were allocated to three groups (Control, Ap80, Ap125), each of 30 at random. Ondansetron 4 mg was injected intravenously to all patients just before the end of surgery. On the morning of surgery, 80 mg and 125 mg aprepitant were additionally administered into the Ap80 group and Ap125 group, respectively. The rhodes index of nausea, vomiting and retching (RINVR) was checked at 6 hr and 24 hr after surgery.
Results
Twelve patients who used steroids unexpectedly were excluded. Finally 78 patients (control : Ap80 : Ap125 = 24 : 28 : 26) were enrolled. Overall PONV occurrence rate of Ap125 group (1/26, 3.9%) was lower (P = 0.015) than the control group (7/24, 29.2%) at 6 hr after surgery. The nausea distress score of Ap125 group (0.04 ± 0.20) was lower (P = 0.032) than the control group (0.67 ± 1.24) at 6 hr after surgery. No evident side effect of aprepitant was observed.
Conclusions
Oral aprepitant 125 mg can be used as combination therapy for the prevention of PONV.
Keywords: Aprepitant, Ondansetron, PONV
Introduction
Postoperative nausea and vomiting (PONV) along with pain are common symptoms following operations and PONV is one of the complications that bother patients. Severe and continued PONV can lead to dehydration and an abnormality of electrolytes, a delayed recovery, and an extension of the period of hospitalization with severe complications such as the rupture of operation sutures and inhalation pneumonia [
1,
2]. Most importantly, PONV must be absolutely prevented in upper gastrointestinal and neurosurgery.
Currently, although many methods are being used to prevent PONV and new drugs have been developed, it is still not possible to completely prevent PONV. In order to prevent PONV, patients who have a high risk factor of PONV must first be identified; drugs that do not increase the risk of PONV when administered must be selected and an appropriate method of anesthesiology must be considered so that the possibility of the occurrence of PONV is minimized. In particular, administration of more than two types of anti-emetic drugs is recommended for patients with a high risk factor of PONV [
3]. The effects of dexamethasone, droperidol and the selective antagonists of the 5-HT
3 receptor have been proven and the use of these drugs are being recommended for combination pretreatment. However, droperidol cannot be used for various reasons in Korea, and so it is only possible to use the other two drugs domestically.
Aprepitant blocks neurokinin-1 (NK1) receptors and reveals a anti-emetic effect with a long reaction time. NK1 receptors exist in the central and peripheral nervous system and combine themselves with substance P. It is known that substance P exists in high concentrations in the vomiting center of the brain where it reacts with the NK1 receptors and is involved in the vomiting reflex in the brain and the gastrointestinal tract [
4]. It has been reported that the recently introduced NK1 receptor antagonist has preventive effects on central and peripheral vomiting stimulation in an animal model [
5]. It has been revealed that the NK1 receptor antagonists also effectively prevent PONV in clinical tests and that it has the similar effects of 4 mg of ondansetron [
6]. According to the guidelines for the prevention of chemotherapy - induced nausea and vomiting (CINV), the receptor antagonist aprepitant is recommended as part of the joint administration of drugs for the prevention of CINV [
7].
Thus, the purpose of this study is to reveal the differences in the preventive effects of PONV by comparing patients who had been administered with only ondansetron and in combination with aprepitant. In addition, the side effects of the combination pretreatment of the drugs were observed as well.
Materials and Methods
This study was conducted after obtaining the approval of the Institutional Review Board of our institution and the subjects of the study were patients between the ages of 18 and 65 who were scheduled for rhinolaryngological surgery and would be classified as 1, 2 patients by the American Society of Anesthesiologists. Patients who were pregnant or were administered with perioperative anti-emetics, steroids, antihistamine, or psychiatric drugs were excluded.
The patients were randomly assigned into three groups (Control, Ap80, Ap125). Ondansetron 4 mg was applied in the control group, 4 mg of ondansetron and 80 mg of aprepitant were applied together in the Ap80 group and 4 mg of ondansetron and 125 mg of aprepitant were applied together in the Ap125 group in order to compare the effects of aprepitant based on the quantity applied.
80 mg of aprepitant along with 75 mg of ranitidine in the Ap80 group and 125 mg of aprepitant along with 75 mg of ranitidine in the Ap125 group were administered on the morning of the operations. Only 75 mg of ranitidine was administered in the control group. Computer-generated random allocation was utilized for the random allocation. 1.5-2.0 mg/kg of propofol and 0.25-0.5 mg/kg of remifentanil was utilized to induce anesthesia and 0.6-1.0 mg/kg of rocuronium was used as a muscle relaxant. 3.5-7.0 vol% of desflurane was utilized to maintain anesthesia while 0.05-0.1 µg/kg/min of remifentanil was continuously infused. Immediately before the completion of the operation, all patients were given 30 mg of ketorolac for pain control and 4 mg of ondansetron for PONV prevention. In order to compare the anti-emetic effects of aprepitant, the Rhodes index of nausea, vomiting and retching (RINVR) was examined at 6 hours and 24 hours after the operations.
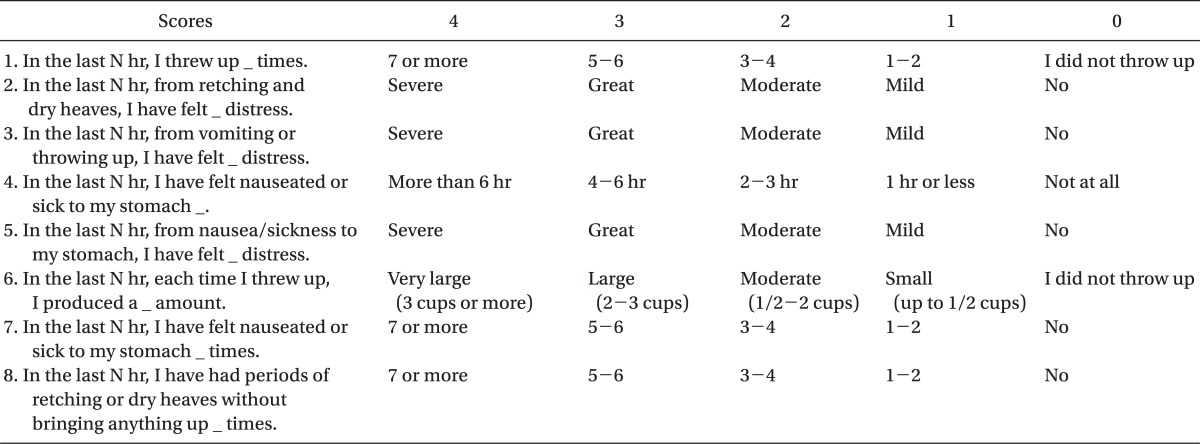
RINVR is a method proposed by Rhodes to objectively assess the degree of nausea distress and vomiting among patients who receive chemotherapy [
8]. The Rhodes survey (
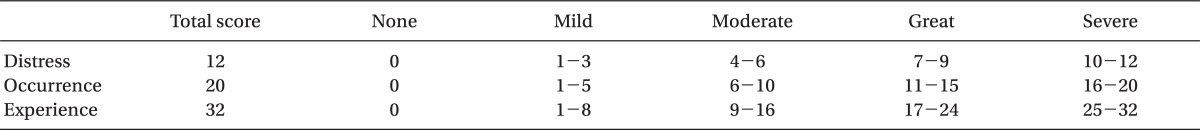
Table 1) is composed of a total of 8 questions and each question has a total of 5 choices. The first choice is selected when the symptom occurs most frequently or when the degree of discomfort is the greatest, and the last choice is selected when there are no symptoms and there is not any degree of discomfort. 4 points are given if the first choice is selected, 3 points for the second choice, 2 points for the third choice, 1 point for the fourth choice and 0 points for the last choice. The points from each of the questions are then calculated. Among the questions of the survey, questions 1, 4, 6, 7 and 8 deals with the subclass of symptom occurrence that has an emphasis on the occurrence of the symptoms and questions 2, 3 and 5 have to do with the subclass of symptom distress that places emphasis on the degree of discomfort of the symptoms. By obtaining the points from the subclass of symptom occurrence of questions 1, 4, 6, 7 and 8 and the points of the subclass of symptom distress of questions 2, 3 and 5 and then adding the points together, the subclass of symptom experience points are obtained. Thus, the total sum of the nausea experience, vomiting experience, and the retching experience points are the RINVR point. Each of the individual RINVR points were assessed using
Table 2 by the 5 stages of cases in which PONV does not occur, mild PONV occurs, moderate PONV occurs, a great amount of PONV occurs, and severe PONV occurs [
9]. Based on these degrees of severity, each group was compared. When patients complained of nausea or vomiting symptoms in the ward and requested that additional treatment be taken, 3 mg of granisetron was injected intravenously after checking the RINVR and, in cases where complaints were made about pain and additional measures were requested to be taken, 1 µg/kg of fentanyl was injected intravenously. In order to examine the side effects of aprepitant, occurrences of symptoms such as depression, chest pain, dyspnea, and hives were ordered to be reported and an observation was made for the same symptoms when making survey rounds.
SPSS (version 17.0) was utilized for statistical verification. The chi square test was utilized for the comparison of the rate of PONV occurrence and the comparison of the number of patients who had received the rescue drug. The one way ANOVA (Bonferroni test) was utilized for the difference in the demographic data of the patients along with a comparison of the RINVR points. Statistical significance was recognized when the P value was below 0.05. G-power 3.0.10 was utilized for calculating the sample size. In the chi square test for the comparison of the rate of occurrence of PONV, the effect size was set to ω = 0.5, α = 0.05, Power (1-β) = 0.95, Df = 2 and a total sample size value of 62 was obtained. In consideration of a rate of elimination of approximately 30%, the testing was conducted on 90 patients with 30 patients from each group.
Results
A total of 78 (Control : Ap80 : Ap125 = 24 : 28 : 26) out of the 90 subject patients, excluding 12 patients who were administered with a steroid during or after the operations, were the final subjects of comparison. The data on the patients and the operative information is shown on
Table 3. There was no significant statistical difference in the comparison of the groups. One patient in the Ap125 group had symptoms of nausea distress after being administered with antibiotics 8 hours after the operation and showed improvement after being administered the rescue drug.
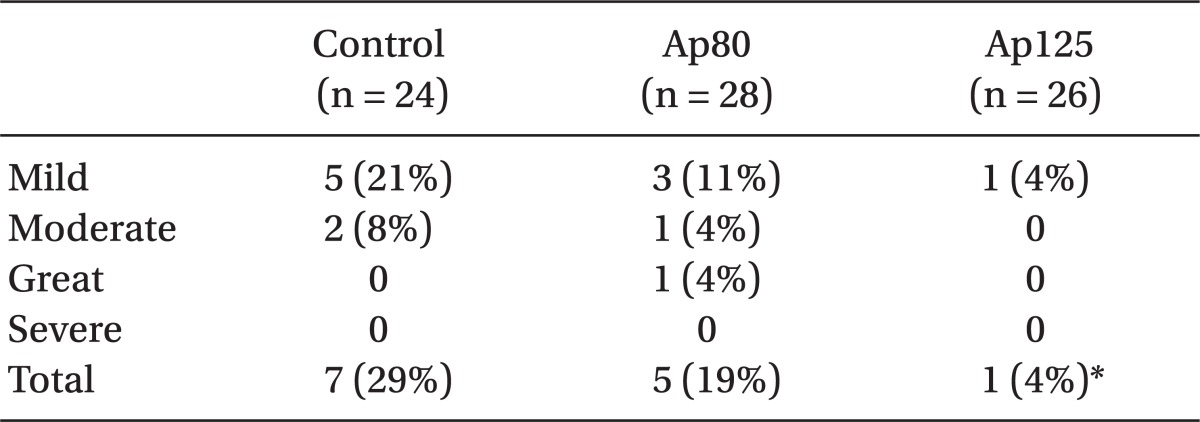
The rate of PONV occurrence measured at 6 hours after the operations were 29.2% (7 out of 24 patients) in the control group, 17.9% (5 out of 28 patients) in the Ap80 group and 3.9% (1 out of 26 patients) in the Ap125 group. There was a significant statistical difference only between the control group and the Ap125 group (P = 0.015), and there was not any significant difference between the other groups. When the PONV was compared based on the degree of severity according to
Table 2, there was not any statistical significance between each of the groups (
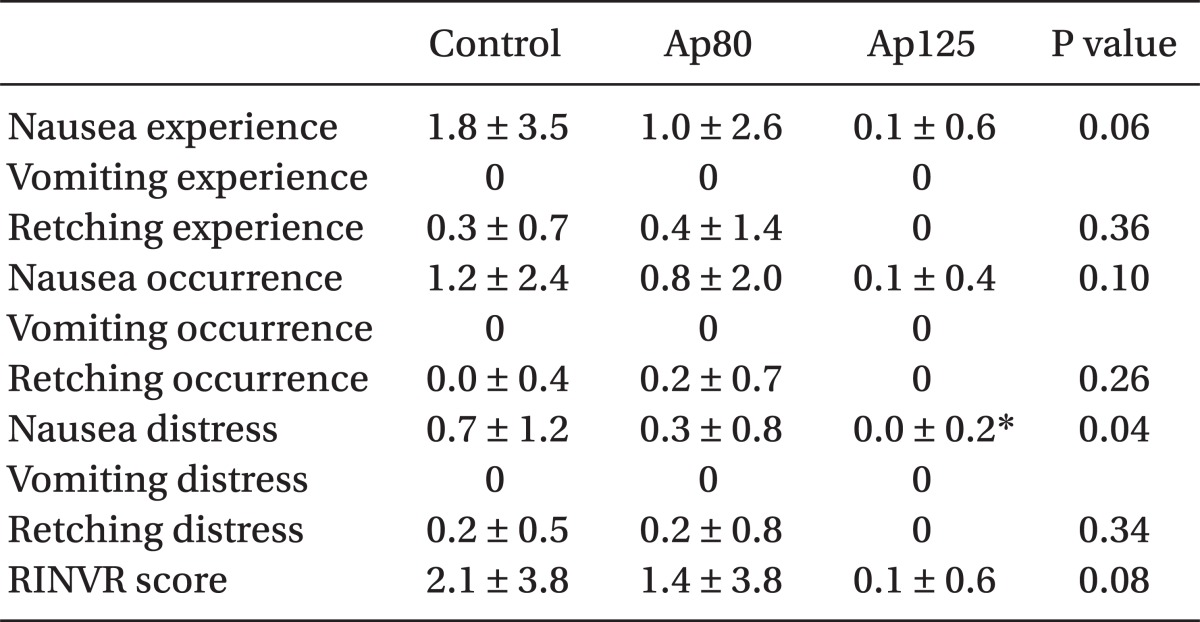
Table 4). 25% (6 out of 24 patients) of the control group, 7.7% (2 out of 28 patients) of the Ap80 group and none of the patients in the Ap125 group were administered with the rescue drug granisetron for PONV treatment within 6 hours after surgery. Also, there was a significant statistical difference (P = 0.011) only between the control group and the Ap125 group. There was no patient who was administered fentanyl additionally to the 30 mg of ketorolac due to severe pain throughout all of the groups. Based on a comparison of the RINVR scores, there was statistical significance (P = 0.032) between the control group (0.67 ± 1.24) and the Ap125 group (0.04 ± 0.20) in nausea distress scores and there was no differences in comparison with other scores (
Table 5). In all groups, there was no patient who complained of PONV between 6 to 24 hours after operations with the exception of 1 patient who complained of nausea distress after the administration of an antibiotic. Furthermore, there were not any symptoms to be considered particularly as being the symptoms of side effects.
Discussion
The NK1 receptor antagonist aprepitant has the effect of preventing CINV and, although it has been reported that a combined administration with a selective antagonist of the 5-HT3 receptor increases this effect, there are not many reports regarding the combination effect for the prevention of PONV. The purpose of this research was to prove the PONV protective effect of a combined pretreatment with aprepitant and ondansetron and its stability.
The rate of occurrence of PONV assessed 6 hours after surgery was lower for patients who were administered with ondansetron and 125 mg of aprepitant compared to the group that was only administered ondansetron. There were also less patients who received the rescue drug in the Ap125 group. This means that the additive administration of aprepitant is effective with regard to the prevention of PONV. Although there was a difference in the overall rate of PONV occurrence, there was not any difference when the frequency was compared after being categorized by degree of severity. Thus, since the subject patients were not placed into a group that have high risk, it is considered that a larger number of patients are needed for this type of comparison.
The NK1 receptor is broadly dispersed on the peripheral and central nerves. It especially exists in the nucleus tractus solitarius that is involved in the vomiting response and the dorsal motor nucleus of the vagus nerve. The stimulation of the NK1 receptor by substance P causes vomiting reflexes and this response can be restrained by the antagonists of this receptor [
10]. When the NK1 receptor antagonist aprepitant was first developed and the results of the clinical tests were announced in 1997 regarding nausea distress and vomiting after the administration of chemotherapy [
11], it became an object of much interest and received the approval of the U. S. Food and Drug Administration in 2006 as a drug that prevents PONV [
6]. Aprepitant very selectively passes through the blood-brain barrier, reacts as the NK1 receptor antagonist, and its half-life of 9-12 hours is very long. There were reports that combined administration of ondansetron with dexamethasone to patients who are receiving a craniotomy did not reveal a difference in the anti-nausea effect, but there was a better anti-vomiting effect [
12]. In addition, it was reported that 40 mg or 125 mg of aprepitant had a better anti-vomiting and effect than 4 mg of ondansetron during the 48 hours after operation on patients who were receiving abdominal operations under general anesthesia. In addition, there was a better stabilizing effect and the QTC interval was not increased as well [
6,
13]. In this study, the additional administration of aprepitant revealed more significance statistically in the anti-nausea effect rather than in the anti-vomiting effect. It is inferred that this is because the subjects of the study were chosen among those who did not have a relatively high degree of risk of PONV and so the rate of occurrence of vomiting and retching in all groups was low. Nevertheless, the result that the additive administration of aprepitant 125 mg with ondanseteron 4 mg can reduce nausea distress after operations among patients who have a low degree of risk of PONV is significant in this study.
Since the 5-HT
3 receptor antagonist was developed and first applied, it has been selected as the ideal drug for nausea distress and vomiting and has been the most used standard drug up to the present for nausea distress and vomiting. Nevertheless, the 5-HT
3 receptor antagonist cannot completely prevent nausea distress and vomiting [
14]. Thus, since it is difficult to prevent PONV with a single method, a diversified approach is required. First, the fundamental cause that creates PONV must be eliminated. Local anesthesia rather than general anesthesia must be performed and total intravenous anesthesia using propofol rather than inhalation anesthetics or nitrous oxide must be utilized. The use of narcotic analgesics for pain control during anesthesia or after operations must be minimized as well. Combined administration of a different type of drug that reacts differently to patients with high risk of PONV is recommended since the effect is increased. The drugs that have had their effect on PONV verified at the present are the 5-HT
3 receptor antagonist, dexamethasone, and droperidol. Two or three combinations of these drugs are recommended. According to a recent report, when comparing the preventive effects of PONV of the NK1 receptor antagonists casopitant and ondanseron after laparoscopic operation, the combined administration of 50 mg, 100 mg and 150 mg of casopitant with ondansetron had more outstanding results than the group in which ondansetron was administered alone [
15]. In addition, it was reported from a comparison of the administration of aprepitant and dexamethasone and the combination of dexamethasone and ondansetron, that aprepitant showed much more of a combination effect than ondansetron [
12]. Thus, it is possible to consider that the NK1 receptor antagonist is effective with regard to PONV in addition to the 3 drugs that have been recommended up until now.
Only 1 out of 26 patients who were additively administered with 125 mg of aprepitant and ondansetron complained of mild PONV, and there was no patient who required an additional rescue drug. This reveals that PONV can be effectively prevented through the combined administration of these two drugs. However, since the subject patients did not have a high degree of risk of PONV, additional research on patients with high risk of PONV is needed. In addition, since all patients in this study, with the exception of one patient, did not complain of PONV 6 hours after the operations, it was useless to observe them past a period of 48 hours. This type of study must be designed additionally on subject patients with many risk factors of PONV. 7 out of 24 patients who were solely administered with ondansetron complained of PONV, and this is not a low incidence. Therefore, there must be consideration given to the combination of drugs of another mechanism for the prevention of PONV for high PONV risk patients.
In conclusion, the rate of occurrence of PONV was lower for patients who were jointly administered with ondansetron and 125 mg of aprepitant compared to the control group that was only administered ondansetron. Furthermore, there were less patients who were given the rescue drug and there were no particular side effects among those who were jointly administered both of these drugs. Therefore, aprepitant 125 mg also regarded as a good pretreatment drug like 5-HT3 receptor antagonist or dexamethasone for the prevention of PONV.
Acknowledgments
This work was supported by research fund of Chungnam National University.
References
1. Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999; 89:652–658. PMID:
10475299.

2. Fisher DM. The "big little" problem of postoperative nausea and vomiting: do we know the answer yet? Anesthesiology. 1997; 87:1271–1273. PMID:
9416707.
3. Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003; 97:62–71. PMID:
12818945.

4. Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS Drugs. 2007; 21:813–833. PMID:
17850171.

5. Tattersall FD, Rycroft W, Cumberbatch M, Mason G, Tye S, Williamson DJ, et al. The novel NK1 receptor antagonist MK-0869 (L-754,030) and its water soluble phosphoryl prodrug, L-758,298, inhibit acute and delayed cisplatin-induced emesis in ferrets. Neuropharmacology. 2000; 39:652–663. PMID:
10728886.

6. Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin MG, et al. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007; 99:202–211. PMID:
17540667.
7. Gralla RJ, Roila F, Tonato M. The 2004 Perugia Antiemetic Consensus Guideline process: methods, procedures, and participants. Support Care Cancer. 2005; 13:77–79. PMID:
15605253.

8. Rhodes VA, McDaniel RW. The Index of Nausea, Vomiting, and Retching: a new format of the lndex of Nausea and Vomiting. Oncol Nurs Forum. 1999; 26:889–894. PMID:
10382187.
9. Suh JK, Bae DJ, Cho SY, Jeon WJ. The assessment of postoperative nausea and vomiting (PONV) using rhodes index in PONV high risk group. Korean J Anesthesiol. 2008; 54:278–282.

10. Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci. 2006; 129:3–16. PMID:
16934536.

11. Kris MG, Radford JE, Pizzo BA, Inabinet R, Hesketh A, Hesketh PJ. Use of an NK1 receptor antagonist to prevent delayed emesis after cisplatin. J Natl Cancer Inst. 1997; 89:817–818. PMID:
9182982.

12. Habib AS, Keifer JC, Borel CO, White WD, Gan TJ. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011; 112:813–818. PMID:
21081776.

13. Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007; 104:1082–1089. PMID:
17456656.

14. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004; 350:2441–2451. PMID:
15190136.

15. Singla NK, Singla SK, Chung F, Kutsogiannis DJ, Blackburn L, Lane SR, et al. Phase II study to evaluate the safety and efficacy of the oral neurokinin-1 receptor antagonist casopitant (GW679769) administered with ondansetron for the prevention of postoperative and postdischarge nausea and vomiting in high-risk patients. Anesthesiology. 2010; 113:74–82. PMID:
20526194.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download