This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Pregabalin is an antiepileptic drug that is effective for treating postoperative pain, neuropathic pain, anxiety, and hemodynamic instability. The aim of this study was to investigate the effect of a single preoperative dose of pregabalin in patients with opioid-induced hyperalgesia (OIH).
Methods
Ninety ASA I-II patients undergoing laparoendoscopic single-site urologic surgery were randomly assigned to one of the following three groups that received either pregabalin or placebo 1 h before anesthesia and an intraoperative remifentanil infusion. Group plL received placebo and 0.05 µg/kg/min remifentanil, group plH received placebo and 0.3 µg/kg/min remifentanil, and group prH received 300 mg pregabalin plus 0.3 µg/kg/min remifentanil. The primary endpoint was pain intensity upon movement 1, 6, 12, and 24 h after surgery. Secondary endpoints were the area of hyperalgesia and mechanical hyperalgesia threshold 24 h after surgery, time to first postoperative analgesic requirement, and cumulative postoperative volume of morphine administered via a patient-controlled analgesia (PCA) pump over 24 h.
Results
The time to first postoperative analgesic requirement in group plH was significantly shorter than that in group plL. The injected PCA volume was significantly greater in group plH than that in the other two groups. Postoperative pain intensity in group plH was significantly greater than that in the other two groups at 6, 12, and 24 h after surgery. The mechanical hyperalgesia threshold and the area of hyperalgesia around the surgical incision 24 h after surgery in group plH differed significantly from those in the other two groups, which were not significantly different. Adverse effects were comparable among groups.
Conclusions
High-dose remifentanil induced hyperalgesia, including increased pain intensity, increased area of hyperalgesia, and decreased mechanical hyperalgesia threshold. These effects were attenuated by oral administration of a single preoperative dose of pregabalin (300 mg) in patients undergoing laparo-endoscopic single-site urologic surgery.
Go to :

Keywords: Opioid-induced hyperalgesia, Pregabalin, Remifentanil
Introduction
Numerous studies have demonstrated that exposure to high doses of remifentanil enhances pain sensitivity, causing opioid-induced hyperalgesia (OIH) [
1-
3]. Shorter acting opioids cause more rapid and frequent hyperalgesia [
4]. Remifentanil is an ultra-short acting µ-opioid receptor agonist that is associated with predictable and rapid recovery independent of dose and duration of infusion [
5].
Pregabalin, a gabapentinoid compound, is an antiepileptic drug that is effective for treating several kinds of pain, including neuropathic, incisional, and inflammatory pain [
6], as well as anxiety [
7] and provides hemodynamic stability [
8]. Pregabalin also attenuates OIH in human and animal studies [
9,
10].
Most studies have evaluated the effect of pregabalin on clinically relevant pain, such as time to first postoperative analgesic requirement, pain intensity, and opioid consumption. Therefore, the present study investigated the effect of a single preoperative dose of pregabalin on hyperalgesia induced by remifentanil for mechanically evoked and clinically relevant pain in patients undergoing laparoendoscopic single-site urologic surgery.
Go to :

Materials and Methods
We obtained approval from our institution's Institutional Review Board and written informed consent from all participants. Ninety-three (n = 31 per group) American Society of Anesthesiologists I-II patients (age, 20-65 years), who were scheduled for laparoendoscopic single-site urologic surgery, were enrolled in this study. Patients were excluded if they had known allergy to gabapentin or pregabalin, had any clinically significant medical or psychiatric conditions, were pregnant or lactating, had a history of alcohol or drug abuse, or were taking opioid-containing pain or sedative medications.
The day before surgery patients were taught how to use the visual analog scale (VAS) and the patient controlled analgesia (PCA) device. They were instructed to self-deliver analgesia whenever they began to feel pain.
All patients were premedicated with midazolam (2-3 mg) before arrival in the operating room. The patients were randomly assigned using a computer-generated random number table into one of the following three treatment groups. Each group received either pregabalin or an identical-looking placebo capsule 1 h before anesthesia and a intraoperative remifentanil infusion. Group plL received placebo and 0.05 µg/kg/min remifentanil, group plH received placebo and 0.3 µg/kg/min remifentanil, and group prH received 300 mg pregabalin plus 0.3 µg/kg/min remifentanil.
The patients were placed on a pulse oximeter, automated blood pressure cuff, electrocardiogram, and end-tidal CO2 devices. In addition, arterial and urinary catheters were placed as part of routine management. Induction of anesthesia was commenced with a slow (30-60 s) intravenous (i.v.) bolus dose of remifentanil (1 µg/kg), followed by propofol (1-2 mg/kg), and tracheal intubation was facilitated with rocuronium (0.9 mg/kg) in all groups. Infusion of remifentanil in all groups was fixed, and anesthesia was maintained with desflurane at an initial end-tidal concentration of 1 minimum alveolar concentration (MAC) and oxygen-air mixture (fraction of oxygen, 50%). Anesthesia levels were monitored during surgery by stepwise titration of the desflurane concentration by 1% volume, based on hemodynamic changes and targeting a bispectral index (BIS) of 40-60.
Upon completion of surgery, neuromuscular blockade was reversed with pyridostigmine (0.2 mg/kg) and glycopyrrolate (0.008 mg/kg) when the train-of-four ratio had returned to 25%. Extubation was performed when BIS values reached 80, and spontaneous breathing was achieved. The remifentanil infusion was discontinued when the final surgical suture had been placed. Each patient was administered analgesics using a PCA pump containing morphine (60 mg), ketorolac (150 mg), and ramosetron (0.6 mg) in normal saline at a total volume of 100 ml. This device was set to deliver a basal infusion of 2 ml/h and bolus doses of 0.5 ml with a 15-min lockout period. Postoperative pain intensity was documented using a 100-mm linear visual analog scale (VAS). The VAS consisted of a straight line with the left end of the line representing no pain and the right end of the line representing the worst pain. Patients were asked to mark the position on the line corresponding to their perception of pain.
The time to first postoperative analgesic requirement, postoperative pain intensity (by VAS), and cumulative postoperative volume injected through the PCA pump over 24 h were recorded. Side effects related to the study drugs included somnolence, dizziness, dry mouth, blurred vision, and postoperative nausea and vomiting (PONV). Nausea or vomiting was treated with intravenous ondansetron (4 mg).
The pain threshold for mechanical punctuate stimuli (mechanical hyperalgesia threshold) was measured preoperatively using Von Frey filaments (Bioseb™, Chaville, France) and was repeated 24 h after surgery on the dominant upper inner arm and peri-incisional areas. This device consists of 20 constant length monofilaments with a stepwise progression in diameter. The numerical grade of the filaments (1.65-6.65) corresponds to a logarithmic function of the equivalent forces of 0.008-300 g. When the tip of a fiber of given length and diameter is pressed against a test area at a right angle, the applied force increases as the probe is advanced, until the fiber bends. After the fiber bends, further advancing of the probe may induce more bending but does not apply more force to the test area. This makes it possible to apply reproducible forces to the tested surface within a wide tolerance range. The force is continuously applied for 1 s and then removed. Subjects were instructed to respond "yes" to indicate that contact was felt during the stimulation or "no" to indicate that contact was not felt during the stimulation. If the subject reported a negative answer, a filament with a larger diameter was used and applied with increasing intensity until the subject reacted. Then, pressure was increased immediately by using a larger filament. The mechanical hyperalgesia threshold was defined as the lowest force (g/mm2) necessary to bend a Von Frey filament, which was perceived to be painful.
The mechanical hyperalgesia threshold was determined with Von Frey filaments on areas 2 cm from the umbilicus (preoperative) and 2 cm from the single port incision site (postoperative) at four points (horizontally and perpendicularly).
Stimulation with a Von Frey filament (number, 6.1; force, 100 g) started from outside the hyperalgesia area, where no pain sensation was experienced, and was then moved toward the incision until the patient reported a distinct change in perception. The area of hyperalgesia for punctuate mechanical stimulation around the surgical incision was determined by testing along linear paths quadrilaterally at a distance of 5 cm around the incision 24 h after surgery. The first point where a painful, sore, or sharp feeling was perceived was marked. If no change in sensation occurred, the stimulation was stopped 1 cm from the incision. A trained anesthesiologist who was not involved in the study assessed clinically relevant and mechanically evoked pain using the Von Frey filaments.
A preliminary investigation showed that the means of the three treatment groups for the mechanical hyperalgesia threshold after surgery were 156, 124, and 148 g/mm2, respectively, with a standard deviation (SD) among subjects of 39.55. Thus, a sample size of 29 patients per group was needed to demonstrate a significant difference with a power of 80% and an α-coefficient of 0.05. Assuming a 5% dropout rate, the final sample size was determined to be 31 patients per group. The results are presented as mean ± SD or percentage of patients. Comparisons of age, body weight, volume percent of desflurane, duration of anesthesia, remifentanil dose administered, mechanical hyperalgesia threshold, area of hyperalgesia, time to first postoperative analgesic requirement, pain intensity, and cumulative postoperative volume injected via the PCA pump for 24 h after surgery among the groups were conducted using one-way analysis of variance. Post hoc comparisons were performed with Bonferroni correction of the significance level. Chi-square tests were used to analyze categorical data such as somnolence, dizziness, blurred vision, dry mouth, and PONV. A P < 0.05 was considered statistically significant.
Go to :

Results
A total of 93 patients were assessed for eligibility and received study medication after randomization. Ninety patients completed the study after two patients underwent conversion to open surgery and one required re-exploration for postoperative bleeding (
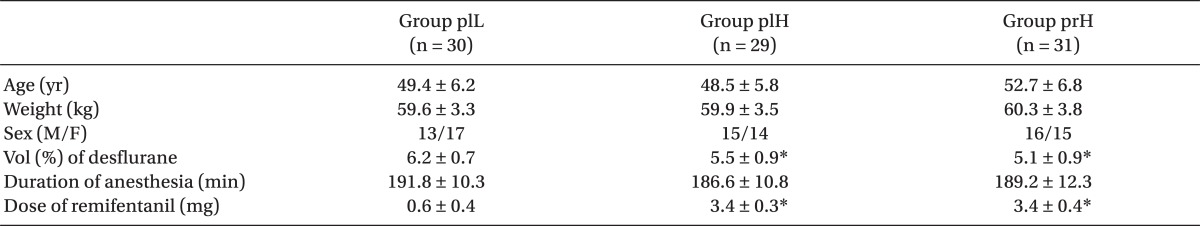
Fig. 1). No significant differences among the three groups were observed with respect to age, weight, sex, or duration of anesthesia. The volume percentage of desflurane was higher in group plL than that in the other two groups (
Table 1).
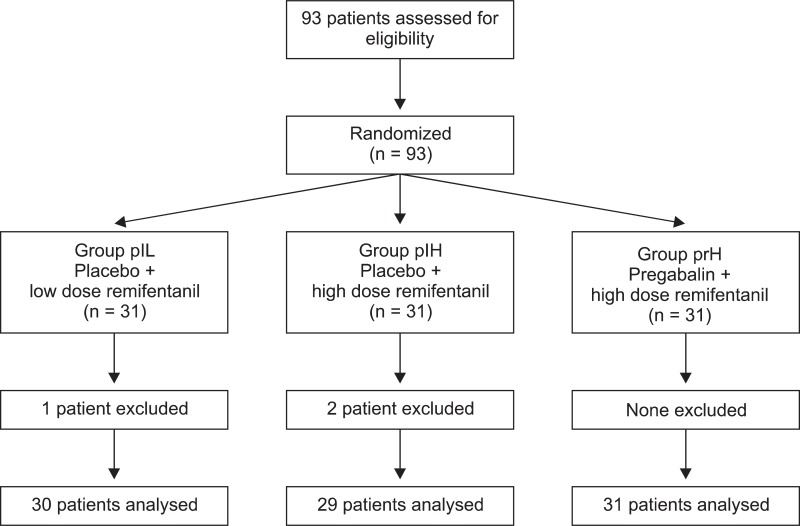 | Fig. 1
|
Table 1

Time to first postoperative analgesic requirement was significantly shorter in group plH than that in group plL. The injected PCA volume containing morphine was significantly greater in group plH than that in the other two groups. The VAS 24 h after surgery revealed significantly higher pain intensity in group plH than that in group plL. The VAS scores 6, 12, and 24 after surgery in group plH were significantly higher than that in group prH. The time to first postoperative analgesic requirement and the VAS 1 h after surgery were not significantly different between group plH and group prH. Preoperative mechanical hyperalgesia threshold did not significantly differ among the three groups. Group plH had a significantly greater area of hyperalgesia and a lower mechanical hyperalgesia threshold compared to those in the other two groups, which were not significant (
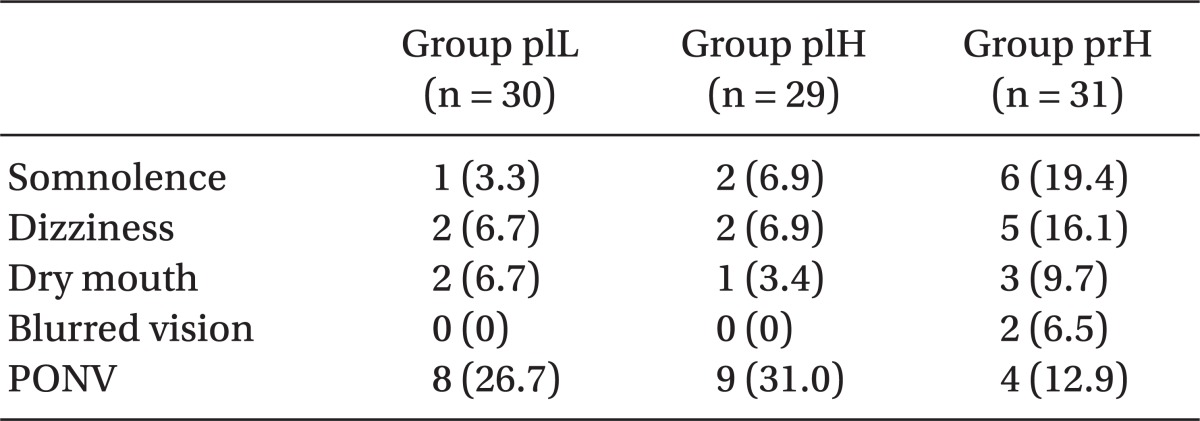
Table 2). In contrast to the peri-incisional area, the preoperative and mechanical hyperalgesia threshold on the forearm did not significantly differ among the groups (data not shown). Side effects were comparable among all groups (
Table 3).
Table 2
Clinically Relevant and Mechanically Evoked Pain
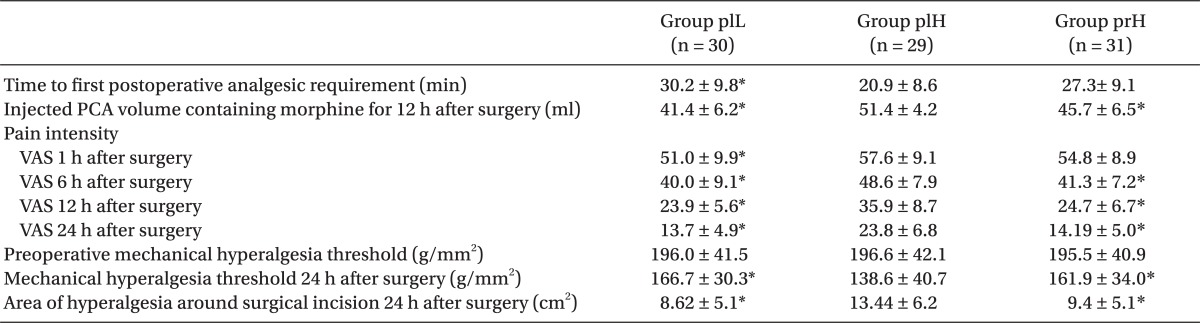

Table 3
Postoperative Side Effects

Go to :

Discussion
A single preoperative dose of pregabalin (300 mg) reduced high-dose remifentanil-induced OIH, including clinically relevant pain and mechanically evoked pain in patients undergoing laparoendoscopic single-site urologic surgery.
OIH is defined as nociceptive sensitization caused by exposure to opioids. This condition is characterized by a paradoxical response in patients receiving high dose or long duration opioids for treating pain. As a result, patients become more sensitive to certain painful stimuli. Prolonged use of opioids is associated with a requirement for increasing doses and the development of abnormal pain. Remifentanil, a µ-opioid receptor agonist, induces long-lasting hyperalgesic effects for days in rodents. In humans, high doses of intraoperative µ-opioid receptor agonists increase postoperative pain and morphine consumption [
1-
3,
9-
11]. In the present study, OIH was defined as an increase in mechanically evoked pain using Von Frey filaments (e.g., an increase in the area of hyperalgesia and a decrease in the tactile pain threshold) in addition to an increase in clinically relevant pain.
Clinically relevant pain measures, including time to first postoperative analgesic requirement, postoperative pain scores by VAS, analgesic consumption, and cumulative postoperative PCA pump-injected analgesic volume represent indirect assessments of OIH. In contrast, mechanically evoked pain measurements modeled by quantitative sensory testing with Von Frey filaments, such as decreased detection thresholds, increased mechanical pain sensitivity, and wind-up ratio as assessed by pinprick devices and algometers, represent direct assessments of OIH in patients under opioid-based anesthesia [
12,
13]. The present study showed that clinically relevant and mechanically evoked pain in group plH, which received high-dose remifentanil, increased significantly compared to those in group plL.
The precise molecular mechanism of OIH is not understood but varies substantially in animal and human studies. OIH is generally thought to result from neurophysiological changes in the peripheral and central nervous system (CNS) that lead to nociceptive sensitization after surgical incision in opioid-treated groups. Extensive internalization and acute receptor desensitization via uncoupling of the receptor from G-proteins, activation of central glutaminergic system, up-regulation of spinal dynorphin, activation of facilitative descending pathways from the rostral ventromedial medulla, genetic influence by the activity of the catecholamine breakdown enzyme catechol-O-methyltransferase, decreased reuptake of neurotransmitters from the primary afferent fibers, and enhanced nociceptive response are suggested as important mechanisms for peri-incisional hyperalgesia induced by high-dose remifentanil used in this study [
4,
14].
Volatile anesthetics, including desflurane, block N-methyl D-aspartate (NMDA) receptors [
15,
16]. However, Hollmann et al. [
15] reported that desflurane inhibits glutamate-induced NMDA receptor responses by only 20% at 0.5 MAC and 40% at 1 MAC. These results suggest that small differences in desflurane concentration do not explain the exaggerated hyperalgesia in patients from group plH who received a high dose of remifentanil.
Strategies for preventing, reversing, or managing OIH have been attempted [
1,
2,
9,
11,
12], and pregabalin has recently emerged as an effective treatment for OIH [
9,
17-
20]. Pregabalin binds potently to the α2δ-1 subunit of voltage-gated calcium channels, which may impair channel trafficking and reduce the release of several neurotransmitters, including glutamate, noradrenaline, serotonin, dopamine, and substance P. This also results in interactions with spino-bulbo-spinal loop-comprising projection neurons in the superficial dorsal horn and brainstem, causing 5-hydroxytryptamine3 receptor-mediated facilitation [
17,
18].
Numerous studies on the efficacy of pregabalin have yielded conflicting results, possibly due to differences in dosage, dosing regimen, or the nature of the surgical procedures [
19]. In the present study, group prH, which received pregabalin, showed attenuated mechanically evoked and clinically relevant pain induced by high dose remifentanil, but were not significantly different from group PlL, which received placebo plus low dose remifentanil. Taken together, pregabalin alone suppressed central sensitization and spinal neuronal hyper-excitability rather than acting as an analgesic. The time to first postoperative analgesic requirement and pain intensity by VAS 1 h after surgery were not significantly different between group plH and group prH; this may be the result of the pharmacokinetics of the analgesic effect of pregabalin. After oral administration of 300 mg pregabalin, sufficient drug concentration in the CNS (peak cerebrospinal fluid concentration) is achieved in 8 h [
21], whereas peak plasma concentration is achieved in 1 h [
17].
Side effects of pregabalin are well tolerated, dose-dependent, and usually transient. In this study, somnolence (19.4%), dizziness (16.1%), dry mouth (9.7%), blurred vision (6.5%), and PONV (12.9%) in group prH, which was administered pregabalin, were not significantly different from the side effects experienced in groups plL and plH, which were not administered pregabalin.
In conclusion, a high dose of remifentanil induced hyperalgesia, including clinically relevant and mechanically evoked pain. This was abrogated by a single preoperative dose of pregabalin (300 mg) in patients undergoing laparo-endoscopic single-site urologic surgery. Further studies are required to investigate the use of pregabalin as part of a multimodal approach for treating OIH.
Go to :

Acknowledgments
This study was supported by Wonkwang Institute of Clinical Medicine in 2012.
Go to :

References
1. Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol. 2011; 61:244–250. PMID:
22025948.

2. Cui W, Li Y, Li S, Yang W, Jiang J, Han S, et al. Systemic lidocaine inhibits remifentanil-induced hyperalgesia via the inhibition of cPKCgamma membrane translocation in spinal dorsal horn of rats. J Neurosurg Anesthesiol. 2009; 21:318–325. PMID:
19955894.

3. Singler B, Tröster A, Manering N, Schüttler J, Koppert W. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg. 2007; 104:1397–1403. PMID:
17513631.

4. Koppert W. Opioid-induced hyperalgesia-Pathophysiology and clinical relevance. Acute Pain. 2007; 9:21–34.

5. Bürkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg. 1996; 83:646–651. PMID:
8780298.
6. Engelman E, Cateloy F. Efficacy and safety of perioperative pregabalin for post-operative pain: a meta-analysis of randomized-controlled trials. Acta Anaesthesiol Scand. 2011; 55:927–943. PMID:
21707548.

7. Wensel TM, Powe KW, Cates ME. Pregabalin for the treatment of generalized anxiety disorder. Ann Pharmacother. 2012; 46:424–429. PMID:
22395254.

8. Rastogi B, Gupta K, Gupta PK, Agarwal S, Jain M, Chauhan H. Oral pregabalin premedication for attenuation of haemodynamic pressor response of airway instrumentation during general anaesthesia: A dose response study. Indian J Anaesth. 2012; 56:49–54. PMID:
22529420.

9. Jo HR, Chae YK, Kim YH, Chai HS, Lee WK, Choi SS, et al. Remifentanil-induced pronociceptive effect and its prevention with pregabalin. Korean J Anesthesiol. 2011; 60:198–204. PMID:
21490822.

10. Ishida R, Nikai T, Hashimoto T, Tsumori T, Saito Y. Intravenous infusion of remifentanil induces transient withdrawal hyperalgesia depending on administration duration in rats. Anesth Analg. 2012; 114:224–229. PMID:
22025495.

11. Chu LF, Cun T, Ngai LK, Kim JE, Zamora AK, Young CA, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by the β-blocker propranolol in humans. Pain. 2012; 153:974–981. PMID:
22365565.

12. Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005; 103:147–155. PMID:
15983467.

13. Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000; 93:409–417. PMID:
10910490.
14. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011; 14:145–161. PMID:
21412369.
15. Hollmann MW, Liu HT, Hoenemann CW, Liu WH, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium. Part II: interactions with volatile anesthetics. Anesth Analg. 2001; 92:1182–1191. PMID:
11323344.

16. Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006; 318:434–443. PMID:
16622040.

17. Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007; 105:1805–1815. PMID:
18042886.

18. Bannister K, Sikandar S, Bauer CS, Dolphin AC, Porreca F, Dickenson AH. Pregabalin suppresses spinal neuronal hyperexcitability and visceral hypersensitivity in the absence of peripheral pathophysiology. Anesthesiology. 2011; 115:144–152. PMID:
21602662.

19. Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011; 106:454–462. PMID:
21357616.

20. Bornemann-Cimenti H, Lederer AJ, Wejbora M, Michaeli K, Kern-Pirsch C, Archan S, et al. Preoperative pregabalin administration significantly reduces postoperative opioid consumption and mechanical hyperalgesia after transperitoneal nephrectomy. Br J Anaesth. 2012; 108:845–849. PMID:
22362672.

21. Buvanendran A, Kroin JS, Kari M, Tuman KJ. Can a single dose of 300 mg of pregabalin reach acute antihyperalgesic levels in the central nervous system? Reg Anesth Pain Med. 2010; 35:535–538. PMID:
20975469.

Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download