Abstract
Background
The authors evaluated the effect of intrathecal mixture of ginsenosides with neostigmine on formalin-induced nociception and made further clear the role of the spinal muscarinic (M) receptors on the activity of ginsenosides.
Methods
A catheter was located in the intrathecal space of male Sprague-Dawley rats. Pain was evoked by injection of formalin solution (5%, 50 µl) to the hindpaw. Isobolographic analysis was done to characterize drug interaction between ginsenosides and neostigmine. The antagonism of ginsenosides-mediated antinociception was determined with M1 receptor antagonist (pirenzepine), M2 receptor antagonist (methoctramine), M3 receptor antagonist (4-DAMP), M4 receptor antagonist (tropicamide). The expression of muscarinic receptor subtypes was examined with RT-PCR.
Results
Intrathecal ginsenosides and neostigmine produced an antinociceptive effect during phase 1 and phase 2 in the formalin test. Isobolographic analysis revealed an additive interaction between ginsenosides and neostigmine in both phases. Intrathecal pirenzepine, methoctramine, 4-DAMP, and tropicamide reversed the antinociception of ginsenosides in both phases. M1-M4 receptors mRNA detected in spinal cord of naïve rats and the injection of formalin decreased the expression of M1 receptor mRNA, but it had no effect on the expression of other three muscarinic receptors mRNA. Intrathecal ginsenosides little affected the expression of all of muscarinic receptors mRNA in formalin-injected rats.
In herbal medicine, ginseng is very widely used as a folk medicine in the eastern countries [1]. Ginsenosides, ginseng saponins, are the main molecules responsible for the actions of ginseng and more than twenty types of different ginsenosides have been identified [2]. According to the behavioral studies, spinal ginsenosides attenuated several types of nociception in animals [3-6]. Several lines of evidence indicated that ginsenosides modulated Ca2+ channels, thereby leading to the antinociceptive effect [7,8]. On the other hand, alpha-2, muscarinic and opioid receptors were insensitive the antinociceptive effect of ginsenosides [7,9]. However, recent studies demonstrated the involvement of alpha-2 and opioid receptors on the antinociceptive action of ginsenosides in the spinal cord [5,6]. Furthermore, a previous study reported the contribution of spinal muscarinic receptors to the activity of intrathecal ginsenosides [10]. Muscarinic receptors play an important role in the modulation of nociception in the spinal cord [11,12]. Five subtypes of muscarinic receptors (M1-M5) were identified and characterized [13-15].
Intrathecal neostigmine reduced various nociceptive states through the action on spinal muscarinic receptors [16-21].
In clinic, the combination of drugs has been generally used because it may provide a decreased dose of one drug or an increased maximum achievable effect.
Therefore, the aim of the present study was to determine the characteristics of the drug interaction between intrathecal ginsenosides and neostigmine in the formalin test, which is characterized by two different nociceptive states, acute nociception followed by a facilitated state. In addition, we sought to further clarify the role of muscarinic receptor subtypes on the antinociceptive effects of ginsenosides at the spinal level.
The studies were reviewed and approved by The Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats weighing 250-300 g were used in all experiments. They were individually kept in a temperature-controlled room (22 ± 0.5℃) in which an alternating 12 h light/dark cycle was maintained. Food and water were sufficiently provided at all times. Under sevoflurane anesthesia, a rat was fitted in a stereotaxic head holder and then a polyethylene-10 catheter was inserted into the subarachnoid space through an incision in the atlantooccipital membrane and advanced caudally 8.5 cm to reach the lumbar enlargement [22]. The exterior end of the catheter was tunneled subcutaneously and externalized on the top of head and sealed with a piece of steel wire. The skin was closed with 3-0 silk sutures. After intrathecal catheterization, rats showing neurologic deficits were euthanized immediately with volatile anesthetics, while normal rats were kept in individual cages. Behavioral studies were performed at least 4-5 days following intrathecal catheterization.
The following drugs were used in this study: ginsenosides, neostigmine bromide (Sigma Aldrich Co., St. Louis, MO, USA), pirenzepine dihydrochloride (Sigma), methoctramine tetrahydrocholoride (Sigma), 4-DAMP (diphenylacetoxy-N-methypiperidine, Sigma), tropicamide (Sigma). Ginsenosides were kindly provided by the Korea Ginseng and Tobacco Research Institute (Daejon, Korea). Ginsenosides were dissolved in dimethylsulfoxide (DMSO). The drugs, except ginsenosides being dissolved in dimethylsulfoxide (DMSO), were dissolved in normal saline. All drugs were intrathecally administered in a volume of 10 µl solution, followed by an additional 10 µl of normal saline to flush the catheter using a hand-driven, gearoperated syringe pump.
The formalin test was carried out as a nociceptive behavioral test [8]. Subcutaneous injection of 50 µl of 5% formalin solution was performed into the plantar surface of the hind paw using a 30 gauge needle. The rat displayed inherent abnormal behavior following formalin injection as follows a rapid and brief withdrawal or flexion of the injected paw. This peculiar behavior was called as flinching response and regarded as a painful response. Such pain behavior was therefore quantified by periodically counting the number of flinching of the injected paw. The number of flinches was counted for 1 min periods from 1 to 2 min, 5 to 6 min and every 5 min from 10 to 60 min. Because of the biphasical pattern of the flinching response after formalin injection, the interval from 0-9 min and the interval from 10-60 min were divided into phase 1 and phase 2 of the formalin test, respectively. Upon completion the test, the rats were euthanized with volatile anesthetics.
After acclimatization for 15-20 min in a restraint cylinder, the rats were randomly allocated into one of the drug-treatment groups. The control study was done using intrathecal DMSO (n = 8) or saline (n = 8), depending on the solvent for the agents. Each rat was used only once. The laboratory worker performing the behavioral test was unaware of drug-treatment of animal.
For examination of the time course and the dose-dependency of the antinociceptive effect of ginsenosides [30 (n = 7), 100 (n = 7), 300 µg (n = 7)] and neostigmine [0.1 (n = 6), 0.3 (n = 7), 1 (n = 7), 3 µg (n = 7)], each agent was intrathecally administered. The formalin test was performed 10 min after drug delivery. The ED50 value (effective dose producing a 50% reduction in control formalin response) for the drugs was calculated separately in each phase.
Isobolographic analysis [9] was used to assess the characteristics of pharmacologic interaction between ginsenosides and neostigmine in the formalin test. This method is based on the comparison of doses determined to be equieffective. At first, each ED50 value was determined from the dose-response curves of agents alone. Next, ginsenosides and neostigmine were intrathecally coadministered at a dose calculated using the ED50 (n = 12) values and fractions [1/2 (n = 13), 1/4 (n = 12) and 1/8 (n = 12)] of ED50 for each drug. The ED50 values of the mixture were calculated from the dose-response curves of the combined drugs, and the combinations were used to plot the isobologram. The isobologram was constructed by plotting the ED50 values of the single agents on the X- and Y-axes, respectively. The theoretical additive dose combination was then calculated. From the variance of the total dose, the individual variances for the combined agents were obtained. Moreover, a total fraction value was calculated to describe the magnitude of the interaction.
The fraction values indicate what portion of the single ED50 value was accounted for by the corresponding ED50 value for the combination. Values near 1 indicate an additive interaction, values greater than 1 indicate an antagonistic interaction and values less than 1 indicate a synergistic interaction. The formalin test was done 10 min after coadministration of two drugs and the pharmacologic characteristics was evaluated in phase 1 and phase 2, respectively.
Exploration of the possible interaction between ginsenosides and muscarinic receptors was conducted. Accordingly, several muscarinic receptor antagonists were intrathecally administered 10 min before the delivery of ginsenosides (300 µg). The muscarinic receptor antagonists used in this study were as follows: M1 receptor antagonist, pirenzepine (20 µg, n = 11); M2 receptor antagonist, methoctramine (30 µg, n = 10); M3 receptor antagonist, 4-DAMP (20 µg, n = 10), M4 receptor antagonist tropicamide (10 µg, n = 10). The kinds and the maximal doses of four muscarinic antagonists were selected based on their lack of significant effect on the control formalin response from the previous studies [21,23,24] and our preliminary experiments. The formalin test was conducted 10 min after administration of ginsenosides. The reversal effect was examined in phase 1 and phase 2, respectively.
The behavioral change of ginsenosides and neostigmine was examined in separate rats (n = 10). They received the highest doses of the two drugs used here, and examined for 60 min after intrathecal administration. Motor functions were assessed by examining the righting and placing-stepping reflexes [21]. The former was evaluated by placing the rat horizontally with its back on the table, which normally gives rise to an immediate coordinated twisting of the body to an upright position. The latter was evoked by drawing the dorsum of either hind paw across the edge of the table. Normally, rats try to put their paws forward into a position for walking. Pinna and corneal reflexes were evaluated with a paper string [21]. Each reflex was evoked by stimulation of the ear canal or the cornea with a paper string. Normal rats spontaneously shaked their heads or blinked, respectively. The change of all reflexes was observed.
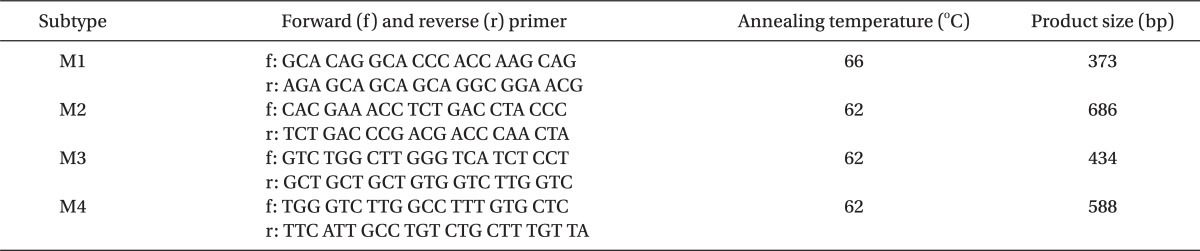
Muscarinic receptor subtypes (M1, 2, 3, and 4) mRNA expression was measured in the dorsal spinal cord of naïve (n = 4), formalin-injected (n = 8), and ginsenosides-delivered rats (n = 8) using reverse transcriptase polymerase chain reaction (RT-PCR). Ginsenosides (300 µg) were administered intrathecally 10 minutes before the formalin test. At 5 and 35 min after formalin injection, rats were killed by decapitation and the spinal cord was quickly removed and stored at -80℃. The area of the spinal cord from L4 to L6 was dissected and total RNA was extracted with RNAiso Plus (Takara Bio, Otsu, Japan) according to the manufacturer's instructions, precipitated with isopropanol, washed with 75% EtOH, air-dried, and dissolved in 20 µl of DEPC-treated water. The yield of the RNA was determined by measuring the absorbance of an aliquot at 260 and 280 nm. RT-PCR was performed using a PrimeScript™ RT-PCR Kit (Takara Bio, Otsu, Japan) according to the manufacturer's protocol. Total RNA (1 µg) was reverse transcribed for 60 min at 42℃. Using HS Prime Taq Premix (GENET BIO, Nonsan, Korea), PCR amplification was performed in 34 cycles of denaturation (95℃ for 60 sec), annealing (see Table 1 for subunit-specific temperatures), and extension (72℃ for 90 sec). Previously published primer sets used for the rat muscarinic receptors were described in Table 1 [25]. β-actin, forward-5'-TCA GGT CAT CAC TAT CGG CAAT-3' reverse-5'-AAA GAA AGG GTG TAA AAC GCA-3' (432 bp). The PCR products were separated on 1.5% agarose gels and visualized by SYBR Safe DNA gel stain. The intensity of the bands was measured by densitometry, and the relative value of the muscarinic receptor subtypes to the β-actin band was calculated in every sample.
Data are expressed as means ± SEM. The time response data are presented as the number of flinching response. The dose-response data are presented as a percentage of the control in each phase. The numbers of flinching response were converted to a percentage of the control in order to calculate the ED50 values of each drug. Percentage of the control = [(Sum of phase 1(2) count with drug) / (Sum of control phase 1(2) count)] × 100. The dose-response data were analyzed using one-way analysis of variance (ANOVA) with Scheffe for post hoc. The dose-response lines were fitted using least-squares linear regression and the ED50, and its 95% confidence intervals were calculated using the method reported by Tallarida and Murray [26]. The difference between theoretical ED50 and experimental ED50, the antagonism of ginsenosides and RT-PCR data were analyzed by t-test. A P < 0.05 was considered significant.
The control groups exhibited a typical biphasic flinching response of the injected paw after the formalin injection. And the sum of the number of flinches did not differ from each other in both phases (saline: DMSO; 18 ± 2: 17 ± 1 in phase 1, 142 ± 6: 143 ± 14 in phase 2).
Fig. 1 exhibits the time course of intrathecal ginsenosides and neostigmine, administered 10 min before the formalin injection. As illustrated, the duration of action of two drugs at the highest dose examined was nearly complete within the entire observation period. Intrathecal ginsenosides and neostigmine produced a dose-dependent reduction the flinching response during phase 1 and phase 2 in the formalin test (Fig. 2 and 3). The phase 1 ED50 values (95% confidence intervals) of ginsenosides and neostigmine were 131.9 µg (60.6-287.7 µg) and 0.8 µg (0.6-1.2 µg), respectively. The phase 2 ED50 values (95% confidence intervals) of ginsenosides and neostigmine were 125 µg (51.3-304.5 µg) and 0.4 µg (0.3-0.5 µg), respectively.
Isobolographic analysis revealed an additive interaction between ginsenosides and neostigmine during phase 1 and 2 in the formalin test (Fig. 4). Accordingly, the ED50 values (95% confidence intervals) of ginsenosides in the mixture of ginsenosides and neostigmine for phase 1 and phase 2 were 56.9 µg (27.4-118.4 µg) and 46.9 µg (38.5-57.2 µg), respectively. These experimental ED50 values were not significantly different from the theoretical ED50 values in both phases. Each total fraction value for the mixture of ginsenosides and neostigmine in phase 1 and phase 2 were 0.86 and 0.75, which indicated an additive interaction.
Intrathecal pirenzepine, methoctramine, 4-DAMP (20 µg), and tropicamide reversed the antinociceptive effect of intrathecal ginsenosides during phase 1 and phase 2 in the formalin test (Fig. 5).
No change of righting, placing-stepping, pinna and corneal reflexes was noted after intrathecal administration of ginsenosides and neostigmine.
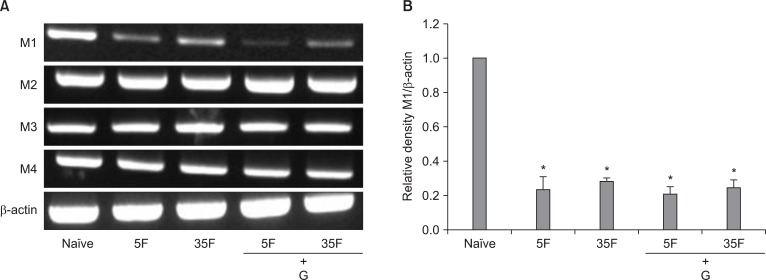
RT-PCR detected the presence of M1-M4 receptor subtypes mRNA in the naive rat lumbar spinal cord. After formalin injection, M1 receptor mRNA levels were decreased, while M2-M4 receptor mRNA levels were not different from those of naive rats (Fig. 6). In addition, no changes were seen on the mRNA levels of all of M1-M4 receptors expressed in formalin-injected rats after intrathecal administration of ginsenosides (Fig. 6A).
In the current study, intrathecal ginsenosides and neostigmine inhibited the phase 1 and phase 2 flinching responses in the formalin test. In the formalin test, the phase 1 flinching response results from the immediate and intense increase of primary afferent activity, which reflects acute pain. On the other hand, the phase 2 response results from the activation of a wide dynamic range of dorsal horn neurons with a very low level of ongoing activity of primary afferent, which reflects a facilitated state. Therefore, these observations suggest that ginsenosides and neostigmine attenuate not only the facilitated state but also acute nociception at the spinal level, which are in agreement with previous studies [5,6,16-21].
On the other hand, an interesting part of this study was the relative effectiveness of drugs. The ED50 values of ginsenosides of phase 1 and phase 2 were similar, while the ED50 value of phase 1 was two-fold higher than that of phase 2 in neostigmine. These findings suggest ginsenosides seems to be equally effective on acute pain and the facilitated state, while neostigmine is much more effective on the facilitated state than on the acute pain.
Ginsenosides has been used in the Far eastern countries to treat various medical illnesses [1]. In particular, it has been shown to exhibit effectiveness against some types of pain in traditional folk medicine. Several animal researches have shown the antinociceptive activity of ginsenosides in the spinal cord. Intrathecal ginsenosides reduced substance P-induced, capsaicin-induced, formalin-induced and postoperative pain behaviors [4-6]. These findings provide a possibility that spinal ginsenosides may be an effective agent in the management of pain in clinics. However, it remains to be determined to date how and where ginsenosides acts to produce antinociceptive action. Previous studies have shown that ginsenosides inhibited voltage-dependent Ca2+ channels in sensory neurons [7,8]. According to a recent study, however intrathecal Korean red ginseng produced a dose-dependent suppression of the flinching response in the rat formalin test and the antinociceptive effect was antagonized by non-selective muscarinic receptor antagonist [10]. In the current study, intrathecal M1-M4 receptor antagonists reversed the effect of ginsenosides. Furthermore, RT-PCR detected M1-M4 receptor mRNA in spinal cord. Previous behavioral and molecular experiments have shown the characteristics between muscarinic receptors and the antinociceptive activity. Intrathecally administered muscarinic agonist produced hot-plate and tail-flick antinociception through the activation of M1 and/or M2 receptors in the spinal cord [11]. Intrathecal muscarinic agonist resulted in strong antinociceptive effect in thermal stimulation, which was exclusively mediated by a combination of M2 and M4 receptors at the spinal level [12]. Electroacupunture-induced antiallodynia on neuropathic pain animal was mediated by spinal M1, but not M2 and M3 receptors [27]. M2 and M4, but not M3 contribute to the modulation of thermal-induced nociception at the spinal level [15]. Autoradiographic study showed the localization of M1 and M2 receptor in substantia gelatinosa of the human spinal cord [13]. Other autoradiographic study demonstrated the existence of M2, M3 and M4, but not M1, receptors in spinal cord [14]. With RT-PCR, M2, M3 and M4 receptors mRNA was present in the spinal cord [15], which is line with the present study. Therefore, these findings jointly suggest that M1-M4 receptors may contribute on the activity of ginsenosides at the spinal level. With respect to the molecular study, considering the antinociceptive property of muscarinic agonists, we expected the increase of the levels of expression of muscarinic receptors mRNA after formalin injection in the current study. However, those of M2-M4 receptors mRNA were little changed after formalin injection. Those observations suggest that the nociception induced by formalin injection is not sufficient to change of M2-M4 receptors mRNA expression or synthesis in the spinal cord. On the other hand, M1 mRNA level was rather decreased after formalin injection. Although this phenomenon has not been exactly explained, these findings suggest that M1 receptor agonist could be less effective to treat pain than M2-M4 receptors agonist. Previous studies have shown no change or different pattern of the expression of some receptors after formalin injection [5,6]. More extensive researches are necessary to investigate those differences. Furthermore, muscarinic receptors mRNA levels measured after formalin injection were not affected by intrathecal ginsenosides, which suggest that ginsenosides themselves have no effect on the expression of muscarinic receptors in the spinal cord.
In the current study, intrathecal neostigmine attenuated phase 1 and phase 2 flinching responses of the formalin test as previous studies [16-21]. Unambiguously, intrathecal neostigmine exerts on muscarinic receptors in the spinal cord and produces an antinociception [21].
According to an isobolographic analysis, intrathecal ginsenosides additively interacted with neostigmine during phase 1 and phase 2 in the formalin test. These findings indicate that spinal ginsenosides cannot potentiate the antinociceptive effect of neostigmine alone in the formalin-induced nociceptive state. This is the first report to describe the property of drug interaction between ginsenosides and neostigmine at the spinal level.
A synergistic interaction is considered likely if basically different mechanisms contribute jointly to the observed actions of the two drugs at a given endpoint, such as antihyperalgesia. However, a synergistic interaction may not be expected if the mechanisms of action of one drug are involved in those of another drug. A previous study has shown that Korean red ginseng inhibited the formalin-induced flinching response and such antinociceptive effect was reversed by non-selective muscarinic receptor antagonist in the spinal cord [10]. Furthermore, in the current study, the antinociceptive effect of ginsenosides was reversed by selective M1-M4 receptor antagonists. These findings suggest that the antinociception of ginsenosides may be mediated by spinal muscarinic receptors. Therefore, ginsenosides and neostigmine may have common pharmacologic sites of action. Based on such observations, ginsenosides may not interact with neostigmine in a synergistic fashion. A previous study has also observed an additive interaction between intrathecal ginsenosides and clonidine [5].
Taken together, intrathecal ginsenosides and neostigmine produced an atinociceptive effect against the formalin-induced nociception and two drugs additively interacted. Additionally, M1-M4 receptors may be involved in the antinociceptive of ginsenosides at the spinal level.
References
1. Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992; 36:27–38. PMID: 1501490.
2. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693. PMID: 10571242.
3. Choi SS, Han EJ, Han KJ, Lee HK, Suh HW. Antinociceptive effects of ginsenosides injected intracerebroventricularly or intrathecally in substance P-induced pain model. Planta Med. 2003; 69:1001–1004. PMID: 14735436.

4. Shin DJ, Yoon MH, Lee HG, Kim WM, Park BY, Kim YO, et al. The effect of treatment with intrathecal ginsenosides in a rat model of postoperative pain. Korean J Pain. 2007; 20:100–105.

5. Yoon MH, Huang LJ, Choi JI, Lee HG, Kim WM, Kim CM. Antinociceptive effect of intrathecal ginsenosides through alpha-2 adrenoceptors in the formalin test of rats. Br J Anaesth. 2011; 106:371–379. PMID: 21169610.
6. Yoon MH, Kim KS, Lee HG, Kim CM, Kim WM, Choi JI, et al. Synergistic interaction between intrathecal ginsenosides and morphine on formalin-induced nociception in rats. J Pain. 2011; 12:774–781. PMID: 21459679.

7. Nah SY, McCleskey EW. Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as mu-type opioids. J Ethnopharmacol. 1994; 42:45–51. PMID: 8046943.
8. Rhim H, Kim H, Lee DY, Oh TH, Nah SY. Ginseng and ginsenoside Rg3, a newly identified active ingredient of ginseng, modulate Ca2+ channel currents in rat sensory neurons. Eur J Pharmacol. 2002; 436:151–158. PMID: 11858794.
9. Shin YH, Jung OM, Nah JJ, Nam KY, Kim CY, Nah SY. Ginsenosides that produce differential antinociception in mice. Gen Pharmacol. 1999; 32:653–659. PMID: 10401990.

10. Kim SY, Yoon MH, Lee HG, Kim WM, Lee JD, Kim YO, et al. The role of adrenergic and cholinergic receptors on the antinociception of Korean red ginseng in the spinal cord of rats. Korean J Pain. 2008; 21:27–32.

11. Iwamoto ET, Marion L. Characterization of the antinociception produced by intrathecally administered muscarinic agonists in rats. J Pharmacol Exp Ther. 1993; 266:329–338. PMID: 8101218.
12. Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, et al. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002; 62:1084–1093. PMID: 12391271.

13. Villiger JW, Faull RL. Muscarinic cholinergic receptors in the human spinal cord: differential localization of [3H]pirenzepine and [3H]quinuclidinylbenzilate binding sites. Brain Res. 1985; 345:196–199. PMID: 3840715.

14. Hoglund AU, Baghdoyan HA. M2, M3 and M4, but not M1, muscarinic receptor subtypes are present in rat spinal cord. J Pharmacol Exp Ther. 1997; 281:470–477. PMID: 9103533.
15. Cai YQ, Chen SR, Han HD, Sood AK, Lopez-Berestein G, Pan HL. Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J Neurochem. 2009; 111:1000–1010. PMID: 19780895.
16. Prado WA, Goncalves AS. Antinociceptive effect of intrathecal neostigmine evaluated in rats by two different pain models. Braz J Med Biol Res. 1997; 30:1225–1231. PMID: 9496442.

17. Yoon MH, Park HC, Kim WM, Lee HG, Kim YO, Huang LJ. Evaluation for the interaction between intrathecal melatonin and clonidine or neostigmine on formalin-induced nociception. Life Sci. 2008; 83:845–850. PMID: 19000700.

18. Yoon MH, Choi JI, Kwak SH. Characteristic of interactions between intrathecal gabapentin and either clonidine or neostigmine in the formalin test. Anesth Analg. 2004; 98:1374–1379. PMID: 15105218.

19. Yoon MH, Choi JI. Pharmacologic interaction between cannabinoid and either clonidine or neostigmine in the rat formalin test. Anesthesiology. 2003; 99:701–707. PMID: 12960556.
20. Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg. 1997; 85:847–853. PMID: 9322468.

21. Yoon MH, Choi JI, Jeong SW. Antinociception of intrathecal cholinesterase inhibitors and cholinergic receptors in rats. Acta Anaesthesiol Scand. 2003; 47:1079–1084. PMID: 12969099.

22. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID: 14677603.

23. Naguib M, Yaksh TL. Antinociceptive effects of spinal cholinesterase inhibition and isobolographic analysis of the interaction with mu and alpha 2 receptor systems. Anesthesiology. 1994; 80:1338–1348. PMID: 8010479.
24. Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg. 1997; 85:847–853. PMID: 9322468.

25. Wei J, Walton EA, Milici A, Buccafusco JJ. m1-m5 muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J Neurochem. 1994; 63:815–821. PMID: 7519660.

26. Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 1987. 2nd ed. New York: Springer-Verlag;p. 1–95.
27. Park JH, Kim SK, Kim HN, Sun B, Koo S, Choi SM, et al. Spinal cholinergic mechanism of the relieving effects of electroacupuncture on cold and warm allodynia in a rat model of neuropathic pain. J Physiol Sci. 2009; 59:291–298. PMID: 19343482.

Fig. 1
Time effect curve of intrathecal ginsenosides (A) and neostigmine (B) for flinching in the formalin test. Each drug was administered 10 min before the formalin injection. Formalin was injected at time 0. Data are presented as the number of flinches. Each line represents means ± SEM of 6-8 rats.
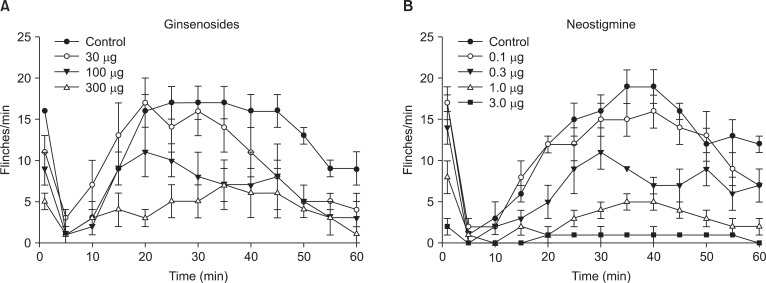
Fig. 2
Dose response curve of intrathecal ginsenosides for flinching in the formalin test. Data are presented as the percentage of control. Ginsenosides produced a dose-dependent inhibition of flinches in phase 1 (A) and phase 2 (B). Each line represents means ± SEM of 7-8 rats. Compared with control, *P < 0.05, †P < 0.01.
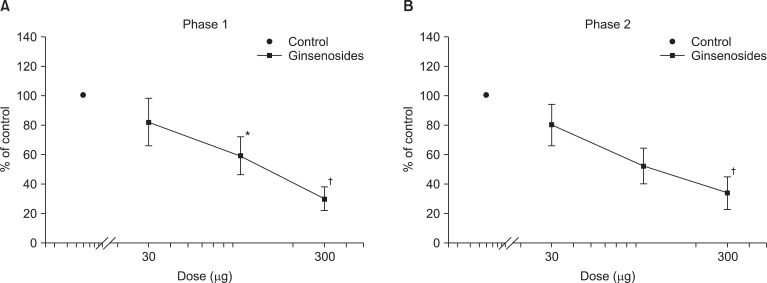
Fig. 3
Dose response curve of intrathecal neostigmine for flinching in the formalin test. Data are presented as the percentage of control. Neostigmine produced a dose-dependent inhibition of flinches in phase 1 (A) and phase 2 (B). Each line represents means ± SEM of 6-8 rats. Compared with control, *P < 0.05, †P < 0.01.
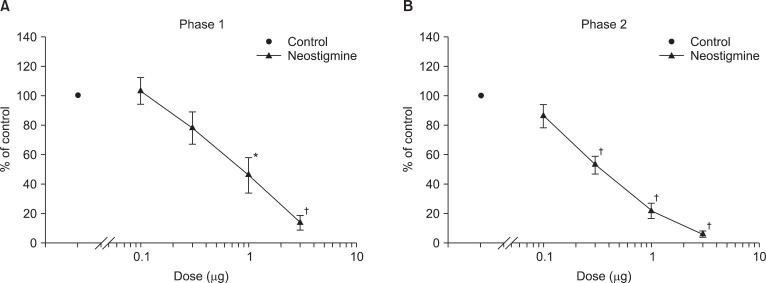
Fig. 4
Isobologram for the interaction between intrathecal ginsenosides and neostigmine during phase 1 (A) and phase 2 (B) in the formalin test. The ED50 values for each agent are plotted on the X- and Y-axes, respectively. Horizontal and vertical bars indicate confidence intervals. The straight line connecting each ED50 value is the theoretical additive line and the point on this line is the theoretical additive ED50. The experimental ED50 point was not significantly different from the theoretical ED50 points, indicating an additive interaction.
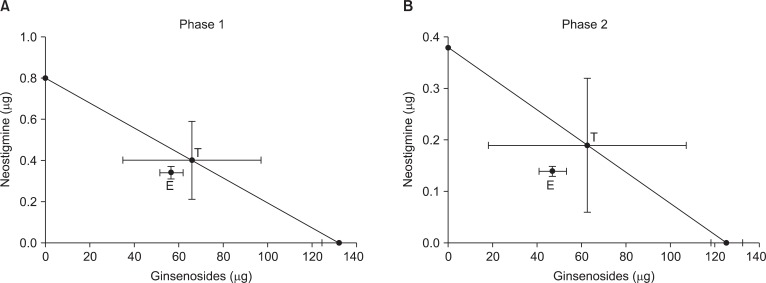
Fig. 5
The effect of intrathecal muscarinic receptor antagonists to intrathecal ginsenosides (300 µg) during phase 1 (A) and phase 2 (B) in the formalin test. Antagonists and ginsenosides were administered intrathecally 20 min and 10 min before formalin injection, respectively. Data are presented as the percentage of control. All of intrathecal muscarinic receptor antagonists reversed the effect of intrathecal ginsenosides in both phases. C: control, G: ginsenosides, M1, M2, M3, M4: antagonists of M1, M2, M3, M4, respectively. Each bar represents as means ± SEM of 5-7 rats. Compared with ginsenosides, *P < 0.05, †P < 0.01, ‡P < 0.001.
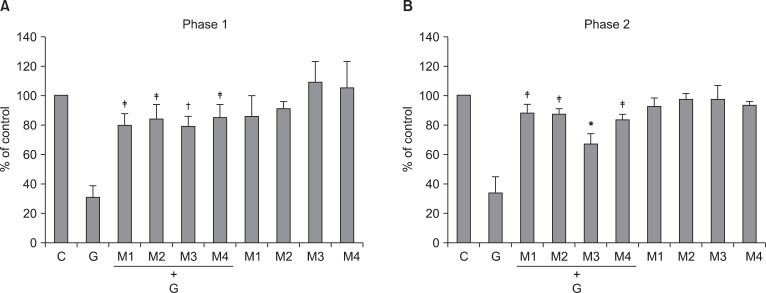
Fig. 6
The expression of muscarinic receptor subtypes (M1-M4) mRNA in the spinal cord of rats. RT-PCR analysis revealed M1, M2, M3, M4 receptors mRNA, and β-actin in naïve state (A). 5F: 5 min after formalin injection, 35F: 35 min after formalin injection, G: ginsenosides. Quantitative analysis indicated that formalin injection reduced the level of expression of M1 receptor mRNA (B), but not the levels of M2-M4 receptor mRNA. Intrathecal ginsenosides did not affect the mRNA levels of all of muscarinic receptors mRNA in formalin-injected rats. Each bar represents as mean ± SEM of 4 rats. Compared with naïve, *P < 0.05.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download