Introduction
Chronic pain is induced by diverse etiology and involves a complex mechanism. One of the important mechanisms is that tissue injury or inflammation induces hyperalgesia resulting from a persistent state of peripheral afferent sensitization that subsequently initiates central sensitization through the release of the excitatory neurotransmitters such as glutamate [
1]. Consequently, single treatment may not be sufficient to successfully alleviate chronic pain. The combination of drugs to treat the chronic pain may have an efficacious effect in suppressing the pain. In a number of animal studies, the combination of drugs that are considered to be involved in different mechanisms have been shown to decrease the pain response effectively, and they have also shown effective results in human studies. For instance, a study conducted by Gilron et al. [
2] suggested that treatment of chronic pain caused by neuropathy with the combination of gabapentin and morphine results in less pain than treatment with a single agent. Therefore, the combination of drugs may result in additivity or synergism and improve efficacy at lower doses compared with the use of one agent alone for treating chronic pain.
In several studies, the antinociceptove effects of reactive oxygen species (ROS) scavengers were examined using the rat formalin test and other chronic pain models. In a study conducted by Wang et al. [
1], the subcutaneous injection of superoxide dismutase mimetics decreased the pain response in a dose-dependent manner in the late phase of the formalin test, and was also shown to inhibit the development of edema induced by carrageenan injection at the hind paw of the rat. Other ROS scavengers such as phenyl-
N-
tert-butylnitrone (PBN) or 4-hydroxy-2,2,6,6-tetramethypiperidine-
N-oxyl (TEMPOL) were also shown to attenuate formalin or capsaicin-induced hyperalgesia when these were injected systemically and intrathecally [
3,
4]. Vitamin E, widely known as a ROS scavenger, is a drug that can be easily obtained and has shown analgesic effects in animal pain models when it is given by both systemic and intrathecal injection in common with the other ROS scavengers [
5,
6]. It is therefore believed that ROS scavengers are efficacious to attenuate peripheral and central sensitization causing chronic pain, and vitamin E may also have antinociceptive effects similar to other ROS scavengers.
Gabapentin is widely used for the treatment of chronic pain, and its effects have been proven in large numbers of animal pain models. In a study conducted by Field et al. [
7], the systemic administration of gabapentin was shown to inhibit the thermal hyperalgesia and tactile allodynia induced by the incision of the plantaris muscle of a rat's hind paw. In other studies, with intrathecal injectins, formalin-induced pain responses were reduced only in the late phase [
8-
10], and synergistic effects were observed when gabapentin was administered intrathecally with other drugs such as N-methyl-D-aspartate (NMDA) receptor antagonists or non-NMDA receptor antagonists [
10,
11]. Thus, these results support the suggestion that gabapentin may have an important role in modulating chronic pain at the spinal level, and may exert an additive or synergistic effect when administrated intrathecally with ROS scavengers.
Therefore, the aim of this study is to investigate the intrathecal interaction between gabapentin and vitamin E used in combination, through the rat formalin test after intrathecal injection.
Go to :

Materials and Methods
All animal tests in this study were reviewed and approved by the Institutional Animal Care and Use Committee of our hospital. Male Sprague-Dawley rats (5-6 weeks old, 180-220 g) were maintained in plastic cages with clean sawdust and with free access to water and food. The animals' room was maintained under a 12/12-hours light/dark cycle, constant temperature (around 23℃) and humidity (around 50%). No more than three rats were placed in each cage.
Using anesthesia produced by enflurane, surgical procedure was performed on rats after skin draping in the lumbar area. Spontaneous breathing was observed under anesthesia. After L5-L6 level was confirmed, a midline incision was performed. L6 spinous process was incised using a surgical microscope and thereafter, the 20-gauge guide needle was advanced to L5-L6 interlaminal space until puncturing the dura mata. Following the guide needle insertion, a polyethylene catheter (PE-10 tube, INTRAMEDIC, Becton Dickinson and Company, Franklin Lakes, NJ, USA) was inserted into the intrathecal space via the guide needle. After entry into the intrathecal space was confirmed by a sudden tail movement, a catheter was situated at the lumbar enlargement of the spinal cord. The catheter was located and fixed in the posterior cervical area through subcutaneous tunneling and the other end of the catheter was plugged. Thereafter, the incision site was sutured. After the rats had completely awoken from anesthesia, only those that had normal motor function were selected and further observation was made for 5-7 days.
The rats were randomized to the gabapentin group, vitamin E group, and combined gabapentin and vitamin E group. In the gabapentin group, 10 µg, 30 µg, 100 µg and 300 µg of gabapentin (1-[aminomethyl] cyclohexanacetic acid; Sigma Chemical Co., St. Louis, MO, USA) were given by intrathecal injection with saline to eight rats for each dose. In the vitamin E group, 3 mg/kg, 10 mg/kg and 30 mg/kg of vitamin E (DL-α-Tocopherol acetate; Sigma Chemical Co., St. Louis, MO, USA) were administrated with olive oil (Sigma Chemical Co., St. Louis, MO, USA) to eight rats for each dose. In the combined gabapentin and vitamin E group, 1/2 dose, 1/4 dose, and 1/8 dose of each drug's ED50 was mixed at a 1 : 1 ratio and administrated.
Gabapentin was dissolved in normal saline and 50 µl was injected using a microinjection syringe through the intrathecal catheter to spread the drugs to the caudal thoracic vertebrae which contain the lumbar enlargement of the spinal cord [
4]. An additional 10 µl of normal saline was administered to flush the catheter. After the drug injection, the end of the catheter was plugged. Vitamin E was dissolved with olive oil and injected in the same manner. The olive oil itself contains a small amount of vitamin E (0.1 mg/ml). However, the total amount of vitamin E in the 50 µl of olive oil is negligible in this study [
5].
Rats were placed in acryl cages for 20 minutes before the start of the test for habituation. Following habituation, the rats received the intrathecal injection and were placed in the cage for another 20 minutes. After 20 minutes intrathecal injection, the rats received 50 µl of 5% formalin injection into the left hind paw using a 25-gauge needle. Flinching behaviors of the left hind paw were recorded every 5 minutes for a total of 60 minutes. After completion of the formalin test, 10 µl of 2% lidocaine was injected through the catheter to observe lower limbs paralysis. Data from rats showing lower limbs paralysis were only used for evaluation, and all rats used for the study were euthanized by exposure to a high concentration of enflurane. Rats exposed to the enflurane showed the suppressed respiratory rate, and then were expired by completely disappeared respiration. If rat's respiration was not suppressed completely, we used the 100% N20 with enflurane.
In the gabapentin group and the vitamin E group, the nociceptive behaviors in the early phase after intrathecal injection did not show any significant difference. Therefore, a dose-response curve for each group was obtained using the average time of flinching only in the late phase. ED50 was defined in this study as the drug dose that reduces 50% of nociceptive behaviors after drug injection. Based on the ED50 from the gabapentin group and the vitamin E group, the drug dose for the combined gabapentin and vitamin E group was determined.
For the injection in the combined gabapentin and vitamin E group, 1/2 dose, 1/4 dose and 1/8 dose of each drug's ED50 were administrated at a 1 : 1 ratio. First, 25 µl of vitamin E dissolved in olive oil was injected through the catheter and 25 µl of gabapentin dissolved in normal saline was injected. Thereafter, 10 µl of normal saline was additionally injected to flush the catheter. Similarly to the gabapentin group and the vitamin E group, eight rats received an injection in the combined gabapentin and vitamin E group and a formalin test was performed in the same manner. ED50 for the combined gabapentin and vitamin E group was obtained based on the formalin test result for the injection of combined drugs.
All data were expressed as mean ± standard error of the mean (SEM). A Tukey post-hoc test was performed using repeated measure ANOVA to compare the nociceptive behaviors between the experimental groups and control groups (saline only or olive oil only group). The ED
50 and confidence interval were obtained using the linear expression program [
12]. SigmaPlot11® software (SYSTAT Software. Inc. Chicago, IL) was used to draw the graphs. To evaluate the interaction between the two drugs, the total dose fraction value was obtained as follows [
13]:
Total dose fraction value = (ED50 value of drug 1 in combination) / ( ED50 value of drug 1 given alone) + (ED50 value of drug 2 in combination) / ( ED50 value of drug 2 given alone)
The total dose fraction value shows the fraction of ED
50 of a single drug injection compared to ED
50 of the combination injection. If a result is close to 1, it indicates that there is additive interaction. If the result is higher or lower than 1, it indicates that there is antagonistic interaction or synergistic interaction. In addition, isobologram was used to analyze the interaction for the combination of the two drugs, which was explained by Tallarida et al. [
14] as a non-mechanistic method of characterizing the effect resulting from the administration of two compounds. Isobologram was drawn by connecting the ED
50 value of vitamin E in the x-axis and the ED
50 value of gabapentin in the y-axis as a linear line. This straight line indicates additivity because the combination of one drug dose and the correspondence of the other drug dose in that line will show the total dose faction value result of 1. This line was employed to distinguish additive from synergistic or antagonistic interaction which was considered as the experimental ED
50 value of the combination that was lower or higher than this line respectively. At this time, because the ED
50 value of the combination was examined at a 1 : 1 ratio, the theoretical additive value, implying that half of the ED
50 value of each drug, was used to evaluate the statistical significance of the experimental ED
50 value [
15]. The statistical difference between the theoretical and experimental values was assessed by a T-test. For all tests, when P values were less than 0.05, the differences were considered statistically significant.
Go to :

Results
After the subcutaneous formalin injection, the behaviors of the rats in the early and late phases were observed. The nociceptive behaviors in the early phase after intrathecal injection of gabapentin were not significantly different to those after the saline injection (
Fig. 1). Similar to the gabapentin group, the vitamin E injection did not give any significant changes to the nociceptive behaviors compared to those after the olive oil injection (
Fig. 2). However, nociceptive behaviors were significantly decreased in the late phase and the pattern was dose-dependent in both gabapentin and vitamin E groups (
Fig. 3). The ED
50 of gabapentin in the late phase was 75.3 ± 9.58 µg, and the ED
50 of vitamin E in the late phase was 17.56 ± 1.65 mg/kg.
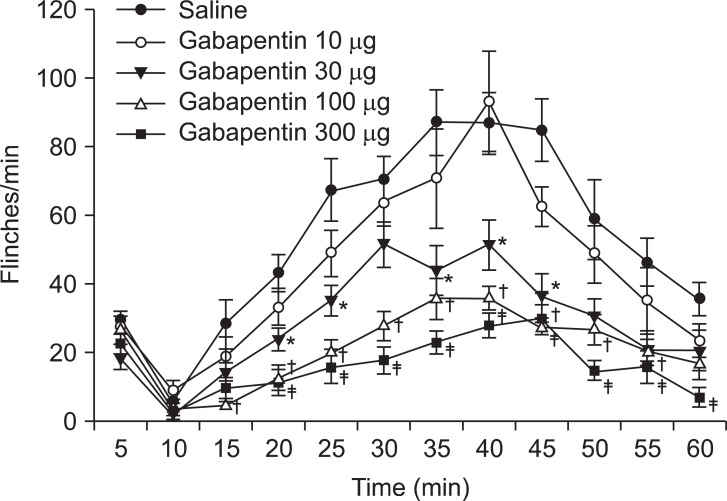 | Fig. 1Time effect curve of intrathecal injection of gabapentin. Numbers of each group were eight. Each point shows the mean ± SE and significant dose-dependent decreases in flinches in the late phase (10-60 min). *P < 0.05, gabapentin 30 µg compared with the saline group. †P < 0.05, gabapentin 100 µg compared with the saline group. ‡P < 0.05, gabapentin 300 µg compared with the saline group. 
|
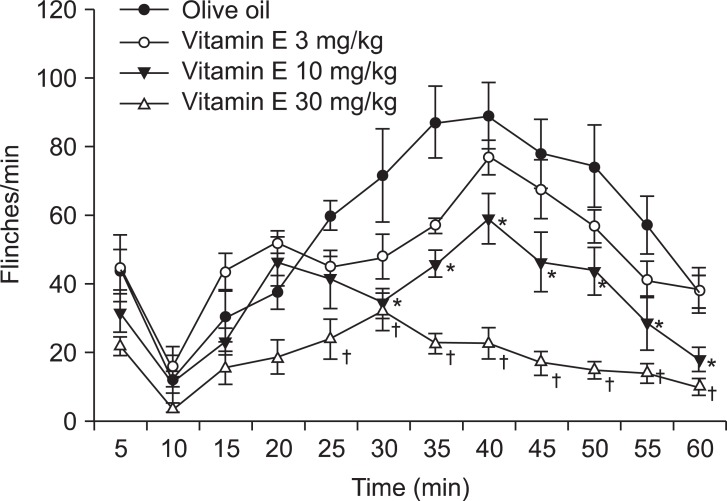 | Fig. 2Time effect curve of intrathecal injection of vitamin E. Numbers of each group were eight. Each point shows the mean ± SE and significant dose-dependent decreases in flinches in the late phase (10-60 min). *P < 0.05, vitamin E 10 mg/kg compared with the olive oil group. †P < 0.05, vitamin E 30 mg/kg compared with the olive oil group. 
|
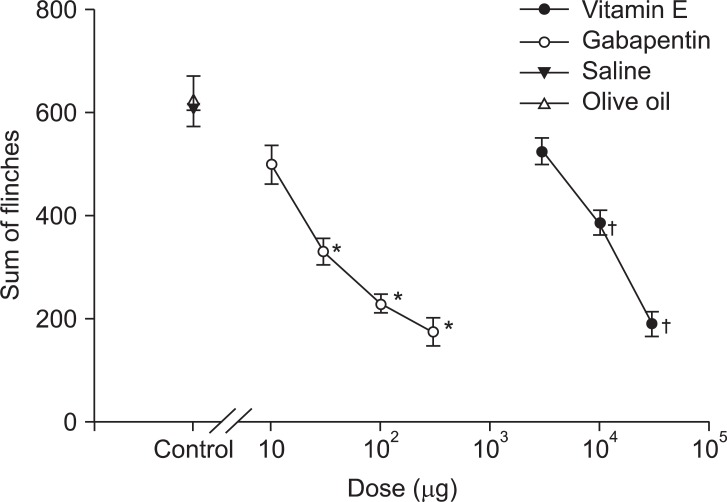 | Fig. 3Dose-response curve of the late phase in an intrathecal single injection of gabapentin and vitamin E. Each point shows the mean ± SE and significant dose-dependent decreases in flinches. *P < 0.05, gabapentin compared with the saline group. †P < 0.05, vitamin E compared with the olive oil group. 
|
The injection of a combination of gabapentin and vitamin E showed significantly decreased nociceptive behaviors in both the early and late phases and the pattern was also dose-dependent (
Fig. 4 and
5). As we could not obtain ED
50 in the early phase for each group, ED
50 for the combination group was not obtained in the early phase. ED
50 was 32.56 ± 4.38 µg for the gabapentin group and 7.82 ± 2.14 mg/kg for the vitamin E group in the late phase. Based on these results, the acquired total dose fraction value was 0.87. In the isobolographic analysis, the value of the experimental ED
50 was smaller than that of the theoretical addictive point, but it was not statistically significant (
Fig. 6).
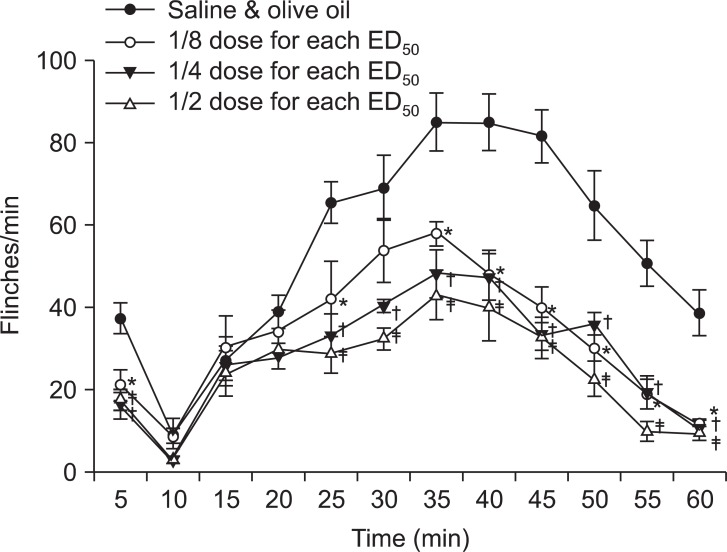 | Fig. 4Time effect curve of the combination of gabapentin and vitamin E. Numbers of each group were eight. Each point shows the mean ± SE and significant dose-dependent decreases in flinches in both early (0-10 min) and late phases (10-60 min). Each ED50, shows the respective ED50 value of intrathecal gabapentin and vitamin E (gabapentin: 75.3 ± 9.58 µg, vitamin E : 17.56 ± 1.65 mg/kg). *P < 0.05, 1/8 dose combination for each ED50 compared with the saline and olive oil combination group. †P < 0.05, 1/4 dose combination for each ED50 compared with the saline and olive oil combination group. ‡P < 0.05, 1/2 dose combination for each ED50 compared with the saline and olive oil combination group. 
|
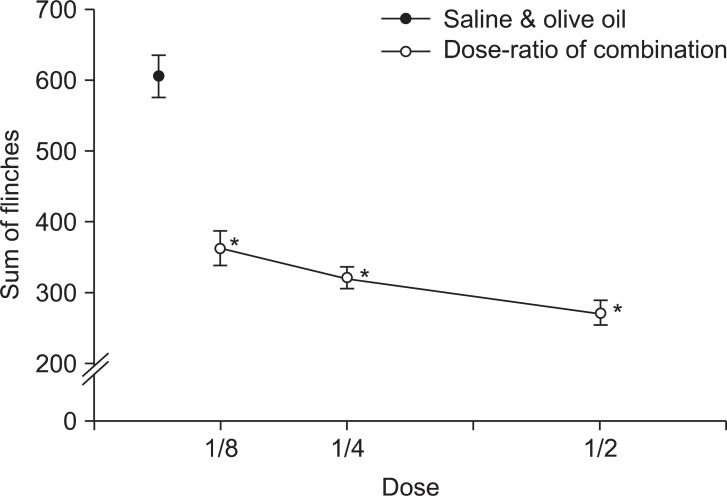 | Fig. 5Dose-response curve of late phase in intrathecal combination of gabapentin and vitamin E. Each point shows the mean ± SE and significant dose-dependent decreases in flinches. *P < 0.05, combination of gabapentin and vitamin E compared with the saline and olive oil combination group. 
|
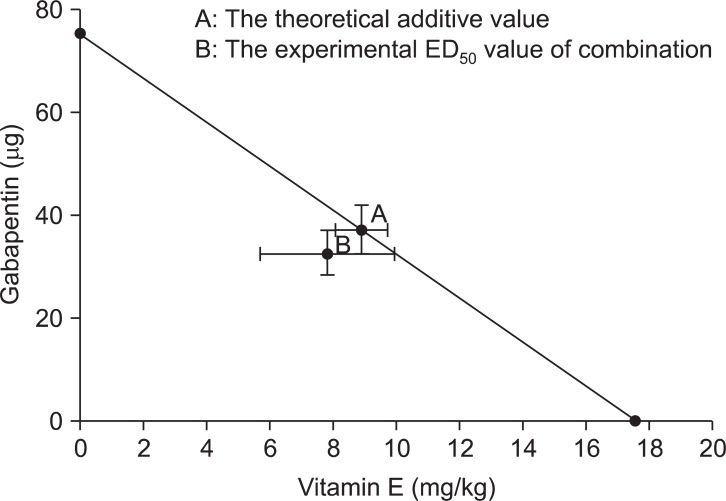 | Fig. 6Isobologram of gabapentin and vitamin E. The experimental ED50 value of the combination was lower than the theoretical additive value in the late phase, but did not show significant difference with the theoretical additive value. 
|
Go to :

Discussion
A previous study regarding formalin concentrations on the nociceptive response in mice showed that the injection of formalin concentrations of 1% or more induced two distinctive periods: an early phase and a late phase [
16]. The early phase seemed to be caused by C-fiber activation that delivered signals to the superficial laminae in the spinal cord and represented the responses for acute nociception lasting 5 minutes directly after the formalin injection. On the other hand, the late phase appeared to be dependent on the combination of an inflammatory reaction in the peripheral tissue and functional changes in the deeper laminae of the spinal dorsal horn due to repetitive nociceptive signals and should imply the responses for formalin-induced hyperalgesia lasted 10 to 60 minutes after the injection [
1,
17].
In our study, significantly decreased dose-dependent nociceptive behaviors were identified in the gabapentin group and the vitamin E group only in their late phases, whereas the combination group showed those patterns in both early and late phases. In other words, the Intrathecal combination of gabapentin and vitamin E was effective in acute nociception as well as in formalin-induced hyperalgesia. However, ED50 for the combination failed to show a significant difference with the theoretical addictive value. This suggests that the combination of these drugs has a more additive than synergistic effect.
In a study by Yoon et al. [
10], several mechanisms for the synergism of drug combination were described as follows. First, drugs may interact by altering the kinetics of each other. One agent may alter the actions of the other agent at the receptor or channel, and thus may lead to synergism. Second, one agent and the other agent may work at separate anatomic sites, causing synergistic effects. Third, synergism may stem from the characteristics of the action of gabapentin. Gabapentin would decrease the release of excitatory neurotransmitters following inflammatory injuries [
18], thus the combination of gabapentin with drugs that inhibit the central sensitization may result in a greater reduction of the transmission of nociceptive signals, which may develop into synergism. Through these mechanisms, it is considered that there are some common pathways in antinociceptive mechanisms involved with these two drugs, and we could therefore hypothesize that vitamin E may be involved in the intracellular signaling cascade activated by some receptors such as voltage-dependent Ca
2+ channels inhibited by gabapentin, and further studies are necessary to elucidate the action mechanisms of ROS and gabapentin in the spinal cord.
Gabapentin is an anticonvulsant drug that is used mainly to control neuropathic pain, and the mechanisms of gabapentin have been considered in a number of studies. Likewise, in our results, intrathecal gabapentin was not effective on acute nociception caused by formalin injection at rat's hind paw in several studies [
8,
19]. In the study conducted by Yoon et al. [
10], the intrathecal injection of NMDA receptor antagonist such as MK801 resulted in a dose-dependent inhibition of flinching responses not only in the late phase but also in the early phase induced by the formalin test; it was therefore suggested that the NMDA receptor may be involved in acute nociception at the spinal level. This belief is supported by the result that the NMDA receptor antagonist blocked the release of substance-P [
20]. Therefore, it is thought that gabapention may not be involved with the NMDA receptor for antinociceptive effect. This idea is consistent with the study conducted by Cheng et al. [
21] suggesting that the NMDA receptor might not be the action target of intrathecal gabapentin in the postoperative pain model, since intarathecal injection of the MK801 failed to attenuate the antiallodynic effect of intrathecal gabapentin in a rat model of postoperative pain. In addition, gabapentin is considered to exhibit an analgesic effect through a separate anatomic site than the NMDA receptor at the spinal level from the result that the intrathecal combination of gabapention and MK801 created the synergism in the rat formalin test [
10].
In a study conducted by Sutton et al. [
22], gabapentin was found to reduce Ca
2+ influx predominantly though N-type Ca
2+ channels in dorsal root ganglion neurons. In other study [
23], the antiallodynic effect of intrathecal gabapentin was reversed by the co-administration of magnesium chloride and ruthenium rad that had been shown to modulate the binding site of the α
2δ subunit of voltage-dependent Ca
2+ channels in a postoperative pain model. Therefore, the antinociceptive effect of gabapentin may be mediated by the interaction with a target such as voltage-dependent Ca
2+ channels, so that such interaction at the presynaptic neurons will inhibit the excitatory neurotransmitter release, and this is supported by the result that suggested gabapentin decreased the release of glutamate induced by the activation of protein kinase C or adenylyl cyclase [
18]. A similar interaction at the postsynaptic neurons may decrease the second messenger action involved in a intracellular signaling cascade.
Normally, ROS protects cells against pathogens and their intracellular level is controlled by various enzymes. However, diseases or illnesses can increase the ROS level in cells because cells lose their ability to control the ROS level or simply overproduce ROS under these conditions. An increased ROS level eventually leads to cell damage such as swelling and necrosis [
24]. The ROS forms as a byproduct from the activation of enzymes such as monoamine oxidase or cyclooxygenase, and there are various types of ROS but three types of ROS are considered to be involved in central sensitization [
25]: 1) superoxide produced by mitochondrial oxidative phosphorylation and cyclooxygenase reaction; 2) hydrogen peroxide (H
2O
2) produced by enzymatic or chemical dismutation; and 3) nitric oxide (NO) produced from L-arginine by NO synthase. Such ROS is produced from various sources in neurons. A previous study showed that Ca
2+-dependent mitochondrial superoxide was generated by the activation of the NMDA receptor [
26]. Based on the study, we can assume that mitochondria play an important role in producing ROS in central sensitization [
25].
ROS is considered to have a critical role in the development of chronic pain. The ROS level of spinal dorsal horn neurons was increased in a spinal nerve ligation model using rats [
27] and appeared to play an important role in inducing and maintaining long-term prolongation considered to be the electrophysiologic basis of central sensitization in the spinal cord [
25]. ROS scavengers such as PBN or TEMPOL reduced the responsiveness of spinal wide dynamic range (WDR) neurons in the capsaicin-induced hyperalgesia model, and the antihyperalgesic effects of PBN were more powerful during intrathecal than during intraventricular or intraperitoneal administration [
4]. In a study conducted by Tiwari et al. [
28], vitamin E also attenuated the mechanical allodynia and hyperalgesia induced by chronic alcohol administration, and its effects might appear by increasing the endogenous antioxidative capacity such as superoxide dismutase or reduced glutathione and protecting against the oxidative stress-mediated release of cytokine (TNF-α and IL-1β) which further activates protein kinase C known to be an important mediator of neuropathic pain. These results showed the crucial role of ROS in central sensitization, and ROS scavengers seem to express their efficacy as counteracting the oxidative stress at an intracellular level because ROS are produced mainly in intracellular mitochondria in central sensitization.
In previous studies, gabapentin was ineffective in the early phase, implying acute nociception [
8-
10]. Likewise, an intrathecal ROS scavenger such as TEMPOL, showed the same results [
3], and vitamin E was also ineffective for acute nociception in our study. However, the injection of two drugs in combination reduced nociceptive behavior significantly in the early phase. Unfortunately, we do not understand how the intrathecal combination of gabapentin and vitamin E interact to attenuate the acute nociception at the spinal level, and it is believed that further evaluations are needed to investigate the effect of the combination of two drugs in acute nociception.
In conclusion, the intrathecal injection of combined gabapentin and vitamin E significantly reduced nociceptive behaviors in the early and late phases of a formalin test in rats. The antinociceptive effects in the late phase are considered to be the results of additive interaction.
Go to :









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download