This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Pain after laparoscopy is multifactorial and different treatments have been proposed to provide pain relief. Multimodal analgesia is now recommended to prevent and treat post-laparoscopy pain. Dexmedetomidine, an α2 agonist, has well-known anesthetic and analgesic-sparing effects. We evaluated the analgesic effect of perioperative dexmedetomidine infusion during laparoscopic cholecystectomy with multimodal analgesia.
Methods
Forty-two patients aged 20 to 60 years old were allocated randomly into one of 2 groups (n = 21, in each). All patients underwent laparoscopic cholecystectomy under multimodal analgesia. The patients in group P received dexmedetomidine 1 µg/kg during 10 min before induction and then 0.5 µg/kg/h continuously until the removal of the gall bladder while the patients in the group C received saline by the same methods as group P. Total analgesic consumption and VAS score were recorded for the first 24 hr.
Results
There were no significant differences in VAS scores between group P and group C during 24 hr after laparoscopic cholecystectomy. VAS scores of group P were lower than that of group C during the 1st hr after operation. The amount of ketorolac required during the 24 hr after the operation was significantly less in group P compared to group C.
Conclusions
The administration of dexmedetomidine during laparoscopic cholecystectomy with multimodal analgesia has minimal benefits on the reduction of the postoperative pain score. The amount of ketorolac requirements during 24 hr after the operation showed significant difference. Dexmedetomidine might be helpful for the postoperative pain after laparoscopic cholecystectomy with multimodal analgesia.
Go to :

Keywords: Dexmedetomidine, Laparoscopic cholecystectomy, Postoperative pain
Introduction
Laparoscopic cholecystectomy (LC) resulted in less postoperative pain and reduction in the need for analgesics, as compared with open cholecystectomy [
1]. Although pain after LC is less intense than that after open cholecystectomy, some patients still experience considerable discomfort during the first 24 h postoperative.
Pain after laparoscopy is multifactorial and different treatments have been proposed to provide pain relief [
2]. Multimodal analgesia is now recommended to prevent and treat post-laparoscopy pain [
3]. In this study, multimodal analgesia consisted of incisional local anesthetics, NSAID, dexamethasone, removing residual carbon dioxide, and postoperative opioid. We reported that such multimodal analgesic methods were effective for post-laparoscopic pain control [
4].
Dexmedetomidine is a highly selective α
2 adrenoceptor agonist that provides sedation, analgesia, and sympatholysis. These characteristics make dexmedetomidine useful anesthetic adjunct during operation. Previous studies report that intravenous has a definitive role in postoperative analgesia through the reduction of opioid consumption [
5]. The analgesic property of dexmedetomidine is less effective than that of opioid, with the clinical implication that dexmedetomidine could not replace the use of opioids [
6]. However, dexmedetomidine might play a part in multimodal analgesia and be able to reduce pain scores through synergistic mechanisms via the alpha-2 adrenergic pathways.
We investigated whether the addition of dexmedetomidine in the multimodal analgesic regimen reduces pain scores after laparoscopic cholecystectomy.
Go to :

Materials and Methods
After the approval of the Institutional Review Board of our hospital, written informed consents were obtained from all patients participating in this study. A total of 42 patients meeting the criteria of the American Society of Anesthesiologists physical status I and II patients, aged 18-60 years, and who were undergoing elective laparoscopic cholecystectomy under general anesthesia were included in the study. The exclusion criteria included the following: a body mass index exceeding 30 kg/m2, allergy to any medications, renal or hepatic insufficiency, neurological or psychiatric diseases, preoperative heart rate < 45 beats/min, antihypertensive medication with clonidine or other α2 adrenergic agonist.
Patients were randomly allocated to the saline (group C) or to the dexmedetomidine group (group P). There were no significant differences in sex ratio, age, weight, height, and duration of surgery and anesthesia between groups (
Table 1). All patients were premedicated with 0.2 mg of glycopyrrolate by intramuscular injection, 1 hour before surgery.
Table 1

On arrival at the operating room, standard monitoring was established and baseline values of heart rate, blood pressure, oxygen saturation and bispectral index (BIS) were recorded. Before induction, dexmedetomidine (Precedex®, Hospira, USA) 1 µg/kg was infused for 10 min and then 0.5 µg/kg/h continuously to group P until the removal of the gall bladder, while group C received saline by the same methods as group P. The values of heart rate, systolic and diastolic pressure, oxygen saturation and BIS were recorded every 5 min after the infusion of dexmedetomidine and just before induction. If heart rate was less than 40 beats/min, atropine 0.5 mg was administered to the patients. If systolic blood pressure was less than 80 mmHg, then ephedrine 10-20 mg was administered to the patients.
General anesthesia was induced with propofol 1.0 mg/kg initially and 10 mg of propofol was given every 30 sec until the absence of eye-opening in response to verbal command and a BIS score under 60. Rocuronium 0.6 mg/kg was given intravenously to facilitate tracheal intubation. Anesthesia was maintained with 50% inspired nitrous oxide combined with sevoflurane at an end-tidal concentration adjusted to maintain BIS values between 40 and 60, and the heart rate and systolic blood pressure within ± 20% of respective baseline values. The patients were ventilated with 50% oxygen keeping the end tidal concentration of carbon dioxide at 35 ± 5 mmHg. After the induction of anesthesia, 30 mg of ketorolac, and 8 mg of dexamethasone were intravenously administered for the postoperative pain control and for the prevention of nausea and vomiting.
All operations were performed by one surgeon who was highly experienced with the standard technique in LC. Before the creation of pneumoperitoneum, 0.25% bupivacaine (3 ml) was infiltrated intracutaneously and subcutaneously at each trocar insertion site. Pneumoperitoneum was achieved with carbon dioxide, and intraperitoneal pressure was maintained below 12 mmHg during surgery. At the end of operation, the carbon dioxide was carefully evacuated through the open trocars by manual compression and residual neuromuscular blockade was antagonized with neostigmine and glycopyrrolate. Patients were transferred to the PACU where blood pressure, pulse, and oxygen saturation were monitored by nurses who were blinded from the patient group assignment.
Before surgery, the patients were instructed to use a visual analogue scale (VAS: 0-10, where 0 = no pain, and 10 = worst possible pain) for pain measurement and assessed at 15, 30 minutes, and at 1, 2, 4, 8, 12, and 24 h intervals after operation by an anesthesiologist who was not involved in the study. When the patient requested analgesics due to pain or the VAS was higher than 4, 30 mg of ketorolac was IV injected first, and 30 minutes later if the VAS was still higher than 4, patients received intravenous tramadol (50 mg) boluses as weak opioid agonists. Thirty minutes after the administration of tramadol, if patients still complained of pain greater than VAS 4, 20 µg intravenous fentanyl was given repeatedly until the VAS was less than 4. Intravenous ondansetron 4 mg was administered to the patients who were suffering from postoperative nausea and vomiting and was repeated if necessary. The patients, who satisfied the PACU discharge criteria, were discharged to the ward.
Sample size calculations were based on the previous study involving multimodal analgesic treatments after elective laparoscopic cholecystectomy [
7]. We considered a reduction in the VAS of 2.0 as clinically significant. A priori, 21 patients were required as the minimum size for each group assuming an α value of 0.05 and a power value of 80%. All of the results were recorded with mean ± standard deviation. Student's t-test was used to compare to the induction doses of propofol and the amount of ketorolac and tramadol given to patients between group P and group C. Repeated measures ANOVA was used for the comparison of dexmedetomidine effect on repeatedly measured VAS, BIS, heart rate, and blood pressure with time. Statistical analyses were performed using MedCalc for Windows, version 9.6 (MedCalc Software, Mariakerke, Belgium) and P values under 0.05 were considered statistically significant.
Go to :

Results
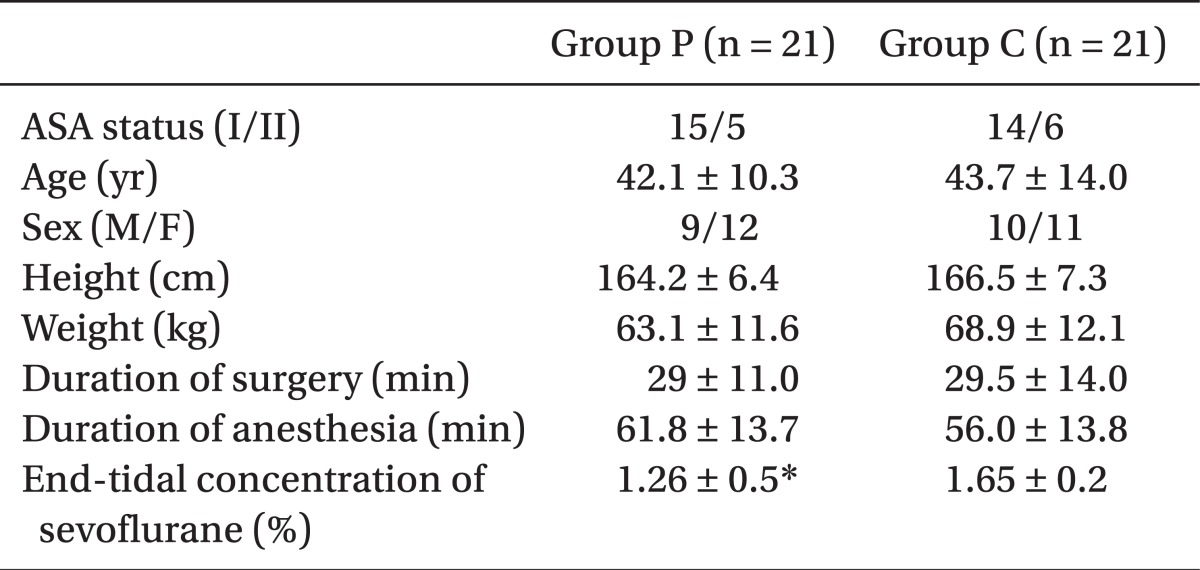
The BIS values for group P decreased significantly compared to group C at 10 min after the infusion of the study solution (dexmedetomidine or saline) (
Fig. 1).
 | Fig. 1Changes in BIS between groups during preoperative infusion of solution. The BIS values of group P decreased significantly compared to group C at 10 min after the infusion of the study solution. Group P: dexmedetomidine infusion during perioperative period, Group C: normal saline infusion during perioperative period. BIS: Bispectral index, T0, 5, 10: solution start time, 5 minutes after solution start, 10 min after solution start. *P < 0.05 compared with group C. 
|
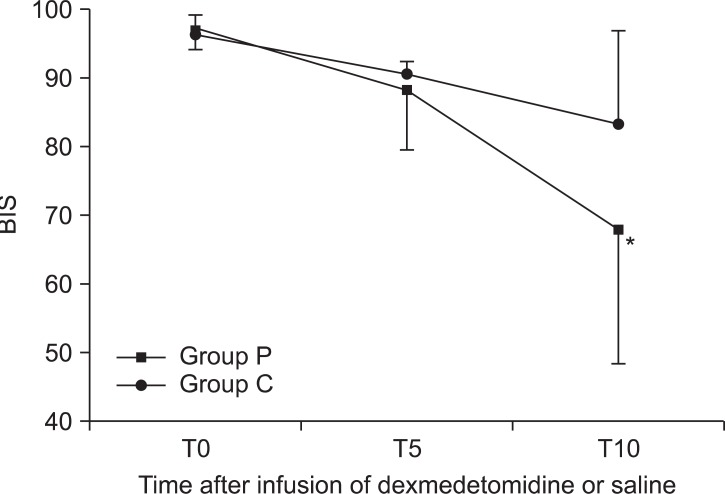
The systolic blood pressure of group P decreased gradually during the infusion of the study solution. The decreases were not so severe that no patients need any vasopressors. There were no significant changes of blood pressure in the group C during the infusion of the study solution (
Fig. 2).
 | Fig. 2Changes in systolic blood pressure between groups during preoperative infusion of solution. The systolic blood pressure of group P decreased gradually during the infusion of the study solution. Group P: dexmedetomidine infusion during perioperative period, Group C: normal saline infusion during perioperative period. T0, 5, 10: solution start time, 5 minutes after solution start, 10 minutes after solution start. *P < 0.05 compared with group C. 
|
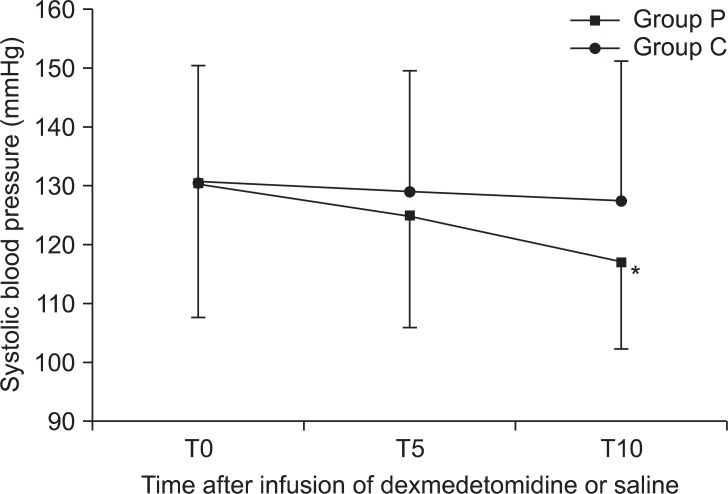
The heart rate of group P decreased gradually during the infusion of the study solution (
Fig. 3). There were less than 50 beats/min in 3 patients, but, no significant hemodynamic changes. There were significant differences in mean end tidal concentrations of sevoflurane during operation (
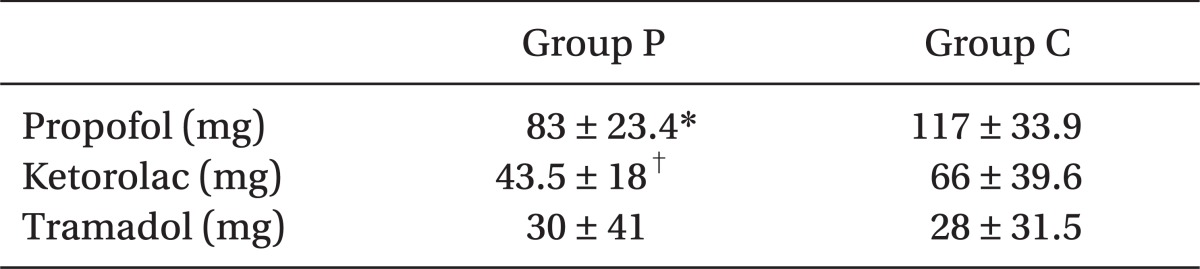
Table 1). The doses of propofol for induction in group P patients were significantly less compared to those of group C (
Table 2).
 | Fig. 3Changes in heart rate between groups during preoperative infusion of solution. The heart rate of group P decreased gradually during the infusion of the study solution. Group P: dexmedetomidine infusion during perioperative period, Group C: normal saline infusion during perioperative period. T0, 5, 10: solution start time, 5 min after solution start, 10 min after solution start. *P < 0.05 compared with group C. 
|
Table 2
Amount of Propofol for Induction and Perioperative Analgesic Consumptions

There were significant differences of VAS between group P and group C until 1 hour after laparoscopic cholecystectomy with multimodal analgesia (
Fig. 4).
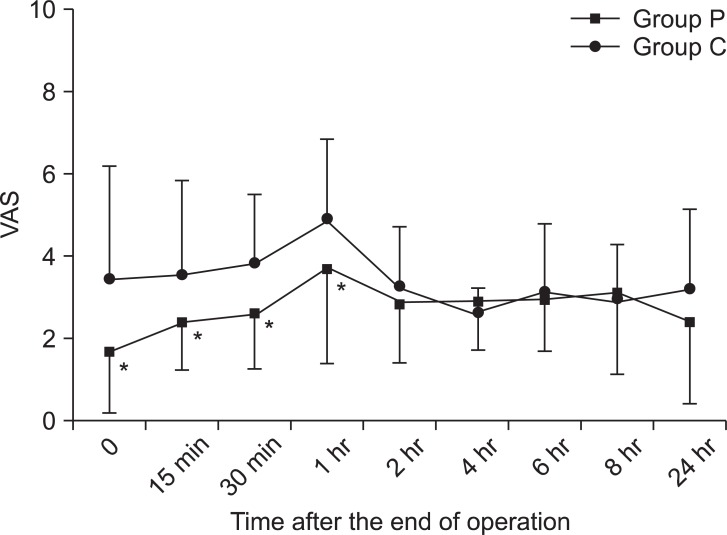 | Fig. 4Changes in postoperative pain between groups. Patients who received dexmedetomidine suffered less pain during 1 hour after laparoscopic cholecystectomy but, not after 1 h after the operation. Group P: dexmedetomidine infusion during perioperative period, Group C: normal saline infusion during perioperative period. VAS: Visual analogue scale. *P < 0.05 compared with group C. 
|
The amount of ketorolac requirements during 24 h after the operation was significantly less in group P compared to group C (
Table 2). None of the patients in group P needed opioid analgesics, but 2 patients in group C needed more potent opioid analgesics for pain control.
Go to :

Discussion
Our study shows that dexmedetomidine significantly reduces pain scores after laparoscopic cholecystectomy with multimodal analgesia for 1 hour but, does not from 1 h to 24 h after the operation.
Postoperative pain in laparoscopy is caused by various reasons, therefore, to reduce it, multimodal treatments are suggested [
8]. There is a report that giving local anesthetic [
9], removing residual carbon dioxide [
8], preemptive analgesia [
10] and dexamethasone [
3] are effective and recently multimodal analgesia using all of methods written above is recommended because it is more effective [
3]. We reported that such multimodal analgesic method was effective for postlaparoscopic pain control [
4].
Dexmedetomidine is a potent and more selective α
2 adrenoreceptor agonist than clonidine. Compared with clonidine, dexmedetomidine has an α
2 : α
1 adrenoreceptor ratio of approximately 1,600 : 1 [
11]. The alpha-2 receptors are located on blood vessels, sympathetic terminals, and central nervous systems, which mediate vasoconstriction, inhibit norepinephrine release, and sedation, reduction of tonic levels of sympathetic outflow, and augmentation of cardiac-vagal activity, respectively [
12].
Dexmedetomidine has sedative and analgesic sparing effects through α
2 adrenoceptor in locus ceruleus [
13]. It is an interesting sedative for not affecting the ventilator response to carbon dioxide [
14]. Unlike other sedatives, it provides respiratory stability in that it does not cause ventilator depression [
15]. The BIS values of group P decreased significantly compared to group C at 10 min after the infusion of dexmedetomidine. But, there was no clinically significant respiratory depression. Although there were no significant differences in VAS between group P and group C from 1 hour after the operation, the amounts of analgesic requirements showed statically significant differences. This result was consistent with other studies [
16]. Ebert et al. [
16] reported that sedation achieved by high plasma concentration recovered 4 h later after discontinuation of dexmedetomidine infusion. This might affect emergence causing extubation delay. As we stopped the infusion after the resection of gall bladder to least affect the recovery, there was no significant difference in extubation time between the 2 groups.
Dexmedetomidine has anesthetic sparing effects during induction and maintenance [
17]. We found that the doses of propofol for induction in the patients of group P were significantly less compared to the doses required of group C. In addition, there were significant differences in the concentrations of inhalational agents between group P and group C.
Dexmedetomidine can cause an increase in blood pressure and a decrease in heart rate with large concentrations or with rapid infusion rates [
18]. Activation of α
2 adrenoceptors on vascular smooth muscle is thought to result in vasoconstriction, increased blood pressure and probable reflex decreased heart rate [
18]. In our study, there were decreases of both heart rates and blood pressure during the bolus infusions. This suggests that initial 1 µg/kg loading infusion for 10 min is not rapid and may not cause large blood concentrations of dexmedetomidine. More central effects, such as sedation, and a decrease in sympathetic outflow and circulating catecholamine might cause decreases in blood pressure and heart rate. Decreases in heart rate were not so severe enough to cause hemodynamic instability.
In this study, we found that the infusion of dexmedetomidine into multimodal analgesia regimen had minimal benefits on the postoperative pain scores after laparoscopic cholecycstectomy without any significant side effects. The amount of ketorolac requirements during 24 hours after the operation showed significant difference. Dexmedetomidine might be helpful for the postoperative pain after laparoscopic cholecystectomy with multimodal analgesia.
Go to :

Acknowledgments
This work supported by the 2011 Inje University Research Grant.
Go to :

References
1. Joris J, Cigarini I, Legrand M, Jacquet N, De Groote D, Franchimont P, et al. Metabolic and respiratory changes after cholecystectomy performed via laparotomy or laparoscopy. Br J Anaesth. 1992; 69:341–345. PMID:
1419439.

2. Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg. 2000; 87:273–284. PMID:
10718794.

3. Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology. 2006; 104:835–846. PMID:
16571981.

4. Lim SH, Jang EH, Kim MH, Cho K, Lee JH, Lee KM, et al. Analgesic effect of preoperative versus intraoperative dexamethasone after laparoscopic cholecystectomy with multimodal analgesia. Korean J Anesthesiol. 2011; 61:315–319. PMID:
22110885.

5. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008; 106:1741–1748. PMID:
18499604.

6. Cortinez LI, Hsu YW, Sum-Ping ST, Young C, Keifer JC, Macleod D, et al. Dexmedetomidine pharmacodynamics: Part II: Crossover comparison of the analgesic effect of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004; 101:1077–1083. PMID:
15505442.
7. Bucciero M, Ingelmo P, Fumagalli R, Noll E, Garbagnati A, Somaini M, et al. Intraperitoneal ropivacaine nebulization for pain management after laparoscopic cholecystectomy: a comparison with intraperitoneal instillation. Anesth Analg. 2011; 113:1266–1271. PMID:
21918162.
8. Alexander JI. Pain after laparoscopy. Br J Anaesth. 1997; 79:369–378. PMID:
9389858.

9. Lee IO, Kim SH, Kong MH, Lee MK, Kim NS, Choi YS, et al. Pain after laparoscopic cholecystectomy: the effect and timing of incisional and intraperitoneal bupivacaine. Can J Anaesth. 2001; 48:545–550. PMID:
11444448.

10. Joshi GP, Viscusi ER, Gan TJ, Minkowitz H, Cippolle M, Schuller R, et al. Effective treatment of laparoscopic cholecystectomy pain with intravenous followed by oral COX-2 specific inhibitor. Anesth Analg. 2004; 98:336–342. PMID:
14742366.

11. Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000; 93:1345–1349. PMID:
11046225.
12. Muzi M, Goff DR, Kampine JP, Roerig DL, Ebert TJ. Clonidine reduces sympathetic activity but maintains baroreflex responses in normotensive humans. Anesthesiology. 1992; 77:864–871. PMID:
1443738.

13. Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996; 84:873–881. PMID:
8638842.

14. Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: Part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004; 101:1066–1076. PMID:
15505441.
15. Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992; 77:1125–1133. PMID:
1361310.
16. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000; 93:382–394. PMID:
10910487.

17. Jalonen J, Hynynen M, Kuitunen A, Heikkilä H, Perttilä J, Salmenperä M, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997; 86:331–345. PMID:
9054252.
18. Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. hemodynamic changes. Anesthesiology. 1992; 77:1134–1142. PMID:
1361311.
Go to :










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download