Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) were first described in 1967 as extensive pulmonary infiltrations accompanied by acute hypoxemia [
1]. Each of these conditions has very high mortality and morbidity rates, and there are no known effective treatments [
2]. Conventional mechanical ventilation (CMV) maintains gas exchange for patients suffering from the above conditions, and has played an important role in the improvement of these disorders. However, large tidal volumes and positive pressure ventilation damage alveolar epithelial cells, pulmonary capillary endothelial cells, and the basal membrane. The effects of CMV cause leakage of endovascular substances and air from the lungs. This eventually leads to alveolar volutrauma, and a vicious cycle of increasing time required on a ventilator, which can exacerbate lung injury and increase the mortality rate [
3,
4].
To solve these problems, the methods involve the lowest possible tidal volume, methods involving permissive hypercarbia, an accompanying respiratory acidosis, and the reversal of inspiration and expiration times. In addition, there are ventilation methods in both research and in practice that can replace CMV and adjuvant ventilation therapies, and these have been evaluated as clinically effective in treating ARDS patients, while reducing ARDS-related mortality rates [
5,
6].
In contrast to CMV, which involves the repetition of expansion and constriction with large tidal volumes, high frequency ventilation (HFV) is performed with a tidal volume that is less than the dead space and respiratory rates that are increased above physiologic levels. Thus, during HFV, lung volumes are kept relatively stable throughout the entire respiratory cycle, and lung injury from heterogenous lung expansion and high airway pressures is reduced, gas exchange is improved, and the inflammatory response is minimized [
7-
9]. Other methods of HFV are also practiced clinically, such as high frequency jet ventilation (HFJV), high frequency oscillation (HFO), and high frequency flow interruption [
10,
11].
When assisting respiration in ALI patients, the application of positive end expiratory pressure (PEEP) minimizes the non-ventilated lung volume and increases functional residual capacity. As a result, PEEP can reduce pulmonary shunting, improve oxygenation, increase oxygen saturation of red blood cells, and reduce inspiratory oxygen requirements [
12].
If a closed respiratory circuit is used for HFJV, the application of PEEP is known to be possible [
13]. The PEEP during HFJV will optimize the lung volume recruitment and improve the oxygenation. But, excessive PEEP is associated with increased risk for lung injury and cardiopulmonary implications.
Using a rabbit model of saline-induced lung injury, we aimed to compare the effect of PEEP during HFJV to the effect of PEEP during CMV on oxygenation, gas exchange, and hemodynamics. We hypothesized that the application of PEEP during HFJV would have the same effect on improving oxygenation as it does during CMV. In addition, we have determined the appropriate level of PEEP improved oxygenation without significantly reducing hemodynamic profiles.
Materials and Methods
Preparation of the laboratory animals
After receiving the approval of the Ethics Committee for Animal Studies, 12 male New Zealand white rabbits weighing 2.8-3.8 kg were randomly divided into groups undergoing either CMV (Group CMV, n = 6) or HFJV (Group HFJV, n = 6). The rabbits were sedated by intramuscular administration of Xylazine (3 mg/kg) (Rumpun®, Bayer, Korea) and Tiletamine/zolazepam (1-2 mg/kg) (Zoletil®, Virbag, Carros, France), in the gluteal muscle. Heating pads were fixed on the surgical table, and a 22 G catheter was placed in the marginal ear vein to secure intravenous access. Normal saline and 6% hydroxyl ethyl starch (Voluven®, Fresineus Kabi, Germany), mixed in a 1 : 1 ratio, were infused continuously at 10 ml/kg/hr. A tracheostomy was performed so that a 4 mm diameter endotracheal tube could be inserted, which was then secured at a depth of 3-4 cm by ligation with surgical thread. During the maintenance of anesthesia, xylazine (2 mg/kg/hr) was administered for sedation, and vecuronium (Norcuron®, Organon, Korea) was bloused (1 mg/kg) and then continuously infused at 1 mg/kg/hr for muscle relaxation. Arterial access was secured with a 22 G catheter in the right femoral artery, which was used for the continuous measurement of arterial pressure and the intermittent collecting of blood. A 4 Fr thermistor-tipped Swan-Ganz catheter (Arrow International Inc., USA) was inserted into the right internal jugular vein for the continuous measurement of pulmonary arterial pressures and core body temperature.
Lung injury
The ALI model by lung lavage was based on the method described by Lachmann et al. [
14]. After disconnecting the endotracheal tube from the ventilator, normal saline (15 ml/kg, heated to 38-39℃) was infused evenly into the lungs through the endotracheal tube. After about 1 min of ventilation using the Harvard respirator (Harvard Apparatus, USA), the infused normal saline was removed by natural drainage or aspiration. Lung lavage by normal saline was then repeated at 5-7 min intervals. Oxygen saturation (SpO
2) was continuously measured from the tongue of the rabbit. When the SpO
2 fell to 90% or below, the lung lavages were stopped, and an arterial blood gas analysis was performed. If oxygen tension decreased to about 100 mmHg, the experiments were preceded to the next steps.
Ventilation method
Group CMV was ventilated with the Harvard respirator. The following settings were used to maintain an arterial carbon dioxide (PaCO
2) of 35-40 mmHg: tidal volume, 8-12 ml/kg; respiratory rate, 20-30 bpm; ratio of inspiratory time to expiratory time (I : E ratio), 1 : 1; and fraction of inspired oxygen (FiO
2), 1.0. In Group HFJV, a 14 G spinal needle with its tip cut off was inserted through the endotracheal tube and connected to the HFJ ventilator (Bromsgrove humidified jet ventilator, Penlon, UK). Ventilation was performed at the following settings: respiratory rate, 120; driving pressure, 2 psi; and FiO
2, 1.0. Lung lavage was performed on both groups 1 h after hemodynamic stability was achieved. After ALI was confirmed, a PEEP of 5 cmH
2O and then a PEEP of 10 cmH
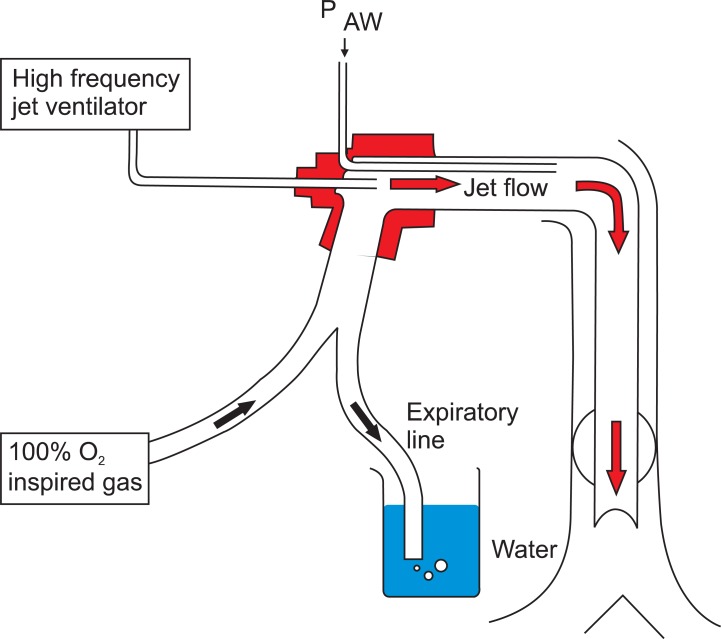
2O were applied to both groups. For the application of PEEP, a 20 cm beaker filled completely with water was placed on an experiment board at the same level as the rabbit. The expiratory port of the ventilator was placed at a depth of 5 cm or 10 cm so that at expiration, the appropriate PEEP would be exerted due to the water (
Fig. 1). Each time PEEP was applied, a 15 min stabilization period was given. After all the results were assessed, the rabbits were euthanized with potassium chloride (KCl) by intravenous administration.
Experiment values
The experiment values were taken at the following 4 points: 1) baseline (B) when the experiment preparations were completed; 2) the point when ALI was confirmed (point ALI); 3) the point after a PEEP of 5 cmH2O was applied (PEEP 5); and 4) the point after a PEEP of 10 cmH2O was applied (PEEP 10). For each group, arterial blood and mixed venous blood gas analyses were performed; the heart rate, mean arterial pressure, pulmonary arterial pressure, core body temperature, pulse oximetry, and peak airway pressures (Paw) were measured; and the alveolar-arterial oxygen tension difference (AaDO2) was calculated.
Statistics
All data are reported as mean ± standard error, and a P value less than 0.05 was considered statistically significant. SPSS (version 12.0, Chicago, IL) was used for statistical analyses. Comparisons within each group were performed with the Friedman's test, and post-hoc testing was done using the Wilcoxon signed rank test. Comparisons of the groups were performed with the Mann-Whitney U test.
Results
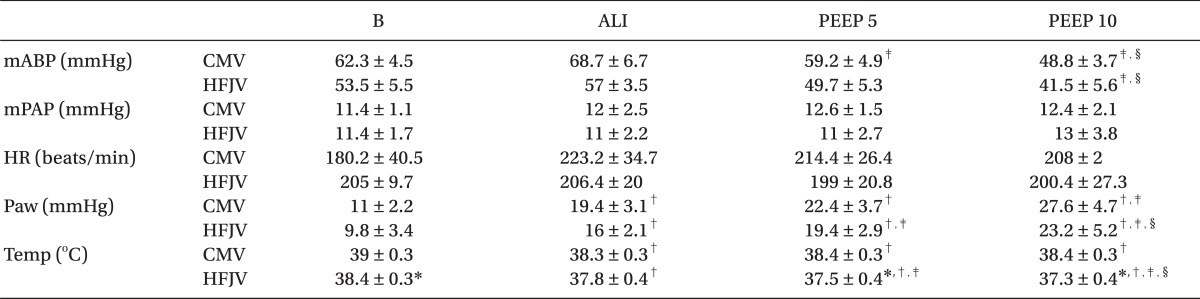
The weights of the rabbits did not differ between groups (Group CMV: 3.1 ± 0.2 kg; Group HFJV: 3.2 ± 0.1 kg). Blood pressure, pulmonary arterial pressure, SpO
2, heart rate, and the arterial oxygen content (PaO
2), taken after the experiment preparations, did not differ between groups. However, both body temperature and PaCO
2 were lower in Group HFJV compared with Group CMV (
Table 1 and
2). Overall, lavage with normal saline was performed 2-4 times, with average volumes of 57 ± 5 ml in Group CMV and 53 ± 3 ml in Group HFJV.
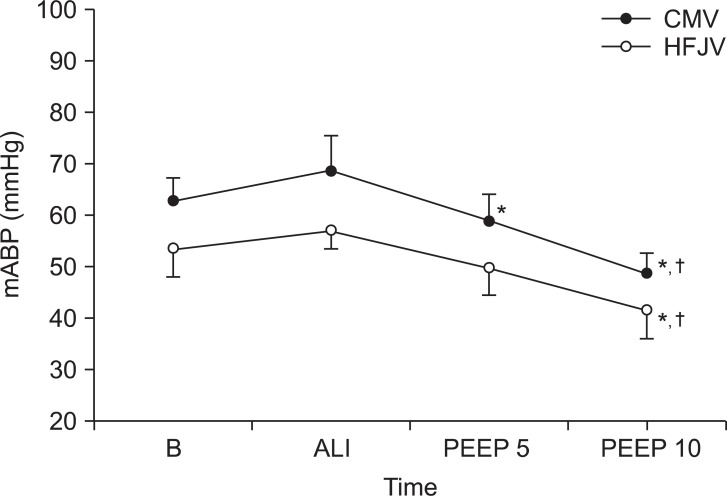
There were initially no differences between the two groups in terms of mean blood pressure; however, the mean blood pressure was progressively reduced for each group after the addition of PEEP. For Group CMV, the mean blood pressure dropped after the addition of 5 cmH
2O of PEEP, and more dropped after the addition of 10 cmH
2O of PEEP. Likewise, in Group HFJV, the mean blood pressure dropped after the addition of 5 cmH
2O of PEEP, and more dropped after the addition of 10 cmH
2O of PEEP (
Fig. 2).
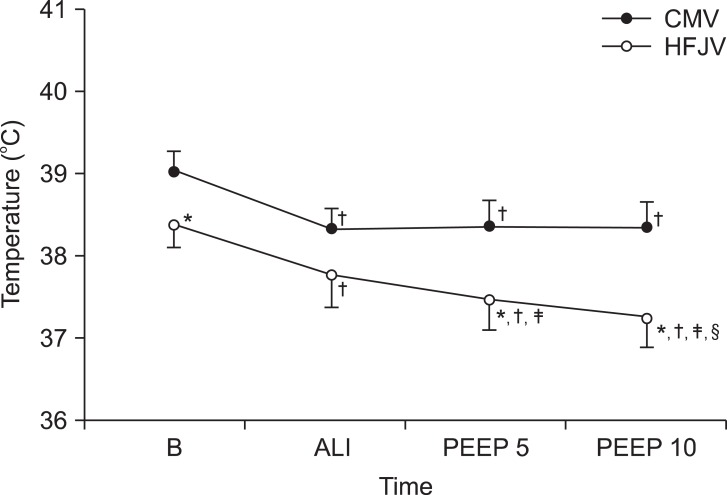
The body temperature in Group CMV was significantly lower than at baseline, and in Group HFJV it became significantly lower at each experimental stage. When the two groups were compared, Group HFJV showed a lower body temperature than Group CMV at baseline, at a PEEP of 5 cmH
2O, and at a PEEP of 10 cmH
2O (
Fig. 3). Otherwise, there were no significant differences between the two groups in terms of pulmonary arterial pressure, SpO
2, or heart rate (
Table 1 and
2).
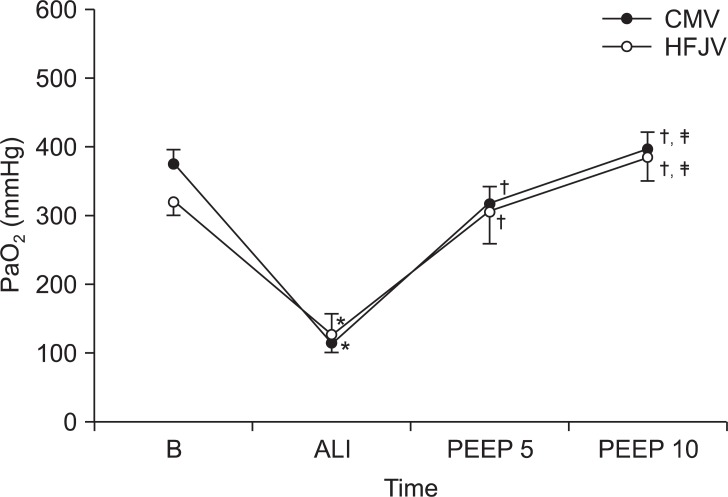
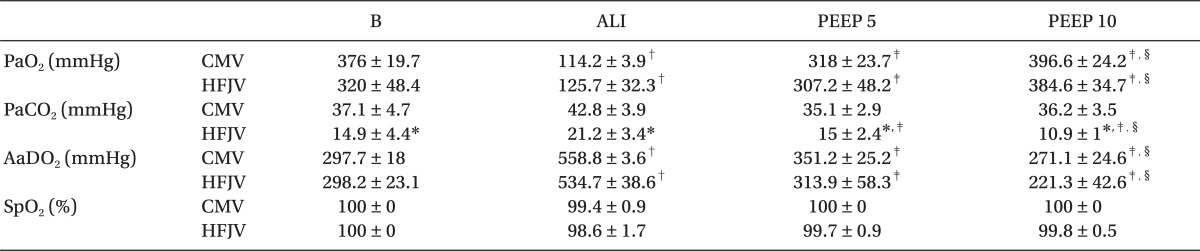
After the lung injury in Group CMV, the PaO
2 initially decreased to 120.1 ± 31.5 mmHg. After the application of PEEP at levels of 5 cmH
2O and 10 cmH
2O, the PaO
2 increased to 328 ± 58.4 mmHg and 409.9 ± 55.4 mmHg, respectively (
Fig. 4). Similarly, after the lung injury in Group HFJV, the PaO
2 initially decreased to 133.8 ± 85.5 mmHg, but after the application of PEEP at levels of 5 cmH
2O and 10 cmH
2O, the PaO
2 rose to 337.3 ± 103.1 mmHg and 388.9 ± 94.3 mmHg, respectively. Although both groups showed a significant difference among PaO
2 levels after lung injury compared to those at baseline, and although PaO
2 levels rose significantly for both groups after PEEP application, there were no differences between the CMV and HFJV groups.
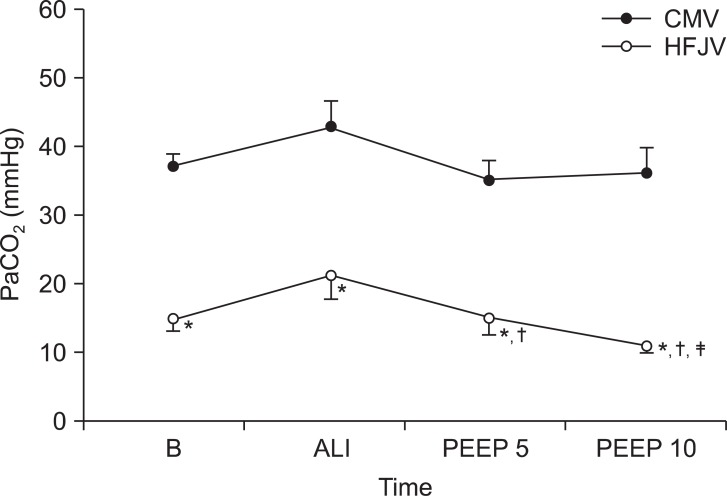
In regards to PaCO
2 levels, there were no significant changes in Group CMV during the experiment. In Group HFJV on the other hand, PaCO
2 levels dropped after the addition of 5 cmH
2O of PEEP, and more dropped after the addition of 10 cmH
2O of PEEP. During the entire experimental period, Group HFJV showed significantly lower PaCO
2 levels than Group CMV (
Fig. 5).
The Paw not only increased significantly in both groups after the lung injury, it also increased at each stage of increased PEEP application (
Table 1). Finally, the AaDO
2 increased in both groups after the lung injury, but decreased after PEEP application, and there were no statistically significant differences between the 2 groups for this parameter (
Table 1).
Discussion
The present study involved the lavage of rabbit lungs with normal saline in order to induce ALI. Gas exchange, oxygenation, and hemodynamic stability after PEEP application during HFJV were compared with those after PEEP application during CMV. Although the PaO2 initially decreased after saline-induced ALI, it clearly increased with the addition of PEEP in both CMV and HFJV groups. Furthermore, hemodynamic stability was maintained for both groups with the addition of 5 cmH2O of PEEP, although blood pressure decreased for both groups upon applying 10 cmH2O of PEEP.
Recent treatments for ARDS are aimed at supplementing oxygenation as much as possible, while preventing further lung injury. The choice of ventilation for ARDS patients requires that which provides the fewest complications, most adequate oxygen supply, and the removal of CO
2 to secure time for recovery from lung injury [
12,
15]. CMV in ALI patients may involve problems, such as high maximum inspiratory capacity and pressure, high mean airway pressure, a decrease in surfactant, and the possibility of mechanical damage from a ventilator [
3]. Therefore, using existing ventilation methods, lung protective modes of ventilation are pursued, which use permissive hypercarbia accompanied by controlled respiratory acidosis, or the reversal of inspiration and expiration times. Moreover, high levels of maximum inspiratory pressure and overexpansion of the lungs can be avoided, but there is controversy over their effects [
5,
16]. For this reason, HFJV and liquid ventilation methods are used to decrease ventilator-associated lung injury [
3].
HFJV, HFO, and high frequency flow interruption are clinically used methods for HFV [
10,
11]. The great advantage of HFV for those with ALI is that the high frequency beats cause turbulent flow, continuous ventilation of the alveoli, and the opening of closed airways. This results in a relative stabilization of lung capacity, the prevention of uneven alveolar expansion and atelectasis, the reduction of intrapulmonary shunt, and an increase in functional residual volume, which allows for adequate oxygenation with low maximum inspiratory pressure [
17,
18]. HFV is known as an effective approach to ALI, because the immune response to lung injury is altered when pressure changes are small and when hemodynamic stability is maintained [
19]. However, there are potential complications with the use of HFV, such as airway damage, necrotic bronchitis, mucous retention, air trapping, air leakage, hemodynamic instability from high airway pressure application, and the hemorrhage of cerebral ventricles in neonates [
20].
HFJV, as used in the present experiment, is a type of HFV that was introduced in 1977 as a treatment modality for ALI, such as those associated with pediatric respiratory distress syndrome and pulmonary air leakage [
19]. HFJV constitutes the use of a high-pressure gas source, pressure-reducing valves with various inspiratory driving pressures, and a small-diameter jet catheter that connects to the respirometer through a small-volume, non-elastic tube. Pressure differences determine the lung volume supplied by the jet ventilator according to the Bernoulli effect, as gas flows through the jet catheter at a very rapid speed. Additionally, expiration is passive, so caution against obstruction around the expiration site is necessary, and adequate humidification is required [
19,
21].
In HFJV, gas exchange depends on: jet speed, mechanical properties of the ventilator, size of the catheter, driving pressure, respiratory frequency, I:E ratio, and PEEP [
22,
23]. Of these, PEEP has been known for some time to increase oxygenation in the setting of acute respiratory failure [
24]. For patients with ARDS, the application of PEEP improves intrapulmonary shunts (despite clinical controversy), assists respiratory muscles by reducing the force needed for respiration, increases functional residual volume, and remobilizes collapsed alveoli to increase oxygenation [
25]. Moreover, PEEP is also known to reduce the loss of surfactant and prevent lung collapse [
26]. After the rabbit lungs were damaged by saline lavage in our study, we found that oxygenation was greatly improved, regardless if they were ventilated by CMV or HFJV. Moreover, the improvement of oxygenation after ALI tended to occur in an additive fashion, whereby more PEEP provided greater increases in PaO
2, though the improvement in PaO
2 was not as pronounced after adding 10 cmH
2O of PEEP, as when adding 5 cmH
2O of PEEP. These findings confirm that PEEP can be properly applied in HFJ ventilation using a closed respiratory circuit.
On the contrary, the side effects of PEEP include elevated thoracic pressures, reduction of venous return to the heart, compromised heart filling, and eventually reduced cardiac output and hypotension. Additionally, these side effects are most pronounced in hypovolemic patients [
27]. Indeed, compared to immediately after pre-lung injury, we demonstrated a drop in blood pressure in Group CMV with the addition of 5 cmH
2O of PEEP after ALI. Furthermore, when a PEEP of 10 cmH
2O was applied, both Group CMV and Group HFJV had clear drops in blood pressure, indicating that greater amounts of PEEP were decreasing blood pressure even more. In addition, HFV is known to cause less intrathoracic pressure changes than CMV, which should maintain hemodynamic stability [
20]. However, we could not identify such an effect in our study, and Group HFJV actually had a lower blood pressure than Group CMV. We explain this observation by noting that Group HFJV had greater driving pressures, and the increase of expiratory lung volume from HFJV was considered to have similar effects on blood pressure as PEEP [
10].
We acknowledge several areas of potential controversy. First, Group HFJV had a lower PaCO2 and body temperature than Group CMV for the entire experimental period. Initially, we had thought that the HFJV applied to the small rabbits was given at an adequate driving pressure and respiration frequency, but instead this may have caused hyperventilation. In fact, the HFJ ventilator that we used is intended for humans as opposed to animal testing, which likely gave greater tidal volumes to the rabbits than we had expected. The further reduce tidal volume would reduces the degree of reduction of mABP and body temperature and increase of Paw is also thought to have been reduced. Second, we may have not induced a severe enough degree of lung injury. There would have been CO2 retention had we made more serious lung injuries, and the benefit to HFJV compared with CMV might have been more easily identified. Third, neither the Harvard respirator nor the HFJ ventilator supports the mechanical application of PEEP. Therefore, in order to apply PEEP, the expiratory port of the ventilator was placed directly into water at depths of 5 cm and 10 cm, yet the expiratory pressure and expiratory gas bubbles made it difficult to maintain and secure the expiratory port at the appropriate depth. However, the expiratory tube had been tightened as much as possible.
We conclude that the application of PEEP in rabbits with ALI effectively improves oxygenation in either HFJV or CMV. Additionally, because 5 cmH2O of PEEP improved oxygenation without significantly reducing blood pressure, we believe that the use of low-level PEEP has a better risk-to-benefit profile than larger amounts of PEEP for the treatment of hypoxemia after ALI, with either HFJV or CMV.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download