Abstract
Background
The activation of guanine nucleotide binding protein-coupled receptors, such as adenosine receptor (ADR) and opioid receptor (OPR), protects the heart against ischemia and reperfusion injury. We hypothesized that ADR or OPR might be involved in polyphenol (-)-epigallocatechin gallate (EGCG)-induced cardioprotection.
Methods
Langendorff perfused rat hearts were subjected to 30 min of regional ischemia and 2 h of reperfusion. Hearts were treated with 10 µM of EGCG, with or without the ADR or OPR antagonist at early reperfusion. Infarct size measured with 2,3,5-triphenyltetrazolium chloride staining was chosen as end-point.
Results
EGCG significantly reduced infarct volume as a percentage of ischemic volume (33.5 ± 4.1%) compared to control hearts (14.4 ± 1.1%, P < 0.001). A nonspecific ADR antagonist 8-(p-sulfophenyl) theophylline hydrate (27.1 ± 1.9%, P < 0.05 vs. EGCG) but not a nonspecific OPR antagonist naloxone (14.3 ± 1.3%, P > 0.05 vs. EGCG) blocked the anti-infarct effect by EGCG. The infarct reducing effect of EGCG was significantly reversed by 200 nM of the A1 ADR antagonist DPCPX (25.9 ± 1.1%, P < 0.05) and 15 nM of the A2B ADR antagonist MRS1706 (29.3 ± 1.7%, P < 0.01) but not by 10 µM of the A2A ADR antagonist ZM241385 (23.9 ± 1.9%. P > 0.05 vs. EGCG) and 100 nM of the A3 ADR antagonist MRS1334 (24.1 ± 1.8%, P > 0.05).
Polyphenol (-)-epigallocatechin gallate (EGCG), a major catechin of green tea, by targeting ischemia [1] and reperfusion [2] provides cardioprotection against ischemia-reperfusion injury. We recently reported that the cardioprotective effect by EGCG was mediated via the ATP-sensitive potassium (KATP) channels [2].
On the other hand, homeostatic regulation and stress responses are mainly regulated by the extracellular signals transduced by guanine nucleotide binding protein (G-protein)-coupled receptor (GPCR) in the heart [3]. Adenosine receptor (ADR) and opioid receptor (OPR) belong to the GPCR family and activation of these upstream receptors might protect the heart by triggering second messengers [4,5].
We hypothesized that ADR or OPR might be activated by EGCG-induced cardioprotection. We therefore investigated the infarct reducing effect with ADR or OPR antagonists in EGCG-induced cardioprotection in isolated rat hearts.
The experimental procedures and protocols used in this study were reviewed and approved by our Institutional Animal Care and Use Committee.
EGCG, 8-(p-sulfophenyl)theophylline hydrate (8-SPT), and 2,3,5-triphenyltetrazolium chloride (TTC) were obtained from Sigma-Aldrich Chemical, St. Louis, MO, USA. Naloxone was purchased from Reyon Pharmaceutical Co., Seoul, Republic of Korea. Fluorescent polymer microspheres were purchased from Duke Scientific, Palo Alto, CA, USA. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo [2,3-a][1,3,5]triazin-5-ylamino] ethyl)phenol (ZM241385), N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy] acetamide (MRS1706), and 1,4-dihydro-2-methyl-6-phenyl-4-(phenylethynyl)-3,5-pyridinedicarboxylic acid 3-ethyl-5-[(3-nitrophenyl)-methyl]ester (MRS1334) were purchased from Tocris Bioscience, Ellisville, MO, USA. Other chemicals were obtained from Sigma-Aldrich Chemical.
EGCG, 8-SPT and naloxone were dissolved in distilled water. DPCPX, ZM241385, MRS1706 and MRS1334 were dissolved in dimethyl sulfoxide. Stock chemicals were stored at -20℃ and were diluted with Krebs-Henseleit (KH) solution to the required final concentrations on the day of each experiment.
Male Sprague-Dawley rats, weighing 280-330 gm obtained from KOATECH Co., Cheongwon-gun, Republic of Korea, were used. They received 50 mg/kg of pentobarbital sodium and 300 IU of heparin intraperitoneally. Hearts were isolated and perfused with modified KH solution containing (in mM) 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 24.8 NaHCO3, 1.2 KH2PO4, and 10 glucose, as described previously [6]. Regional ischemia was induced by pulling the snare which was made at the level of the proximal length of the left coronary artery (LCA) and its major branches and confirmed by regional cyanosis and a substantial decrease in left ventricular developed pressure (LVDP). Reperfusion was started by releasing the snare.
All hearts were subjected to 30 min of regional ischemia and 120 min of reperfusion. Infusion of EGCG and antagonists was started 10 min before the onset of reperfusion and continued for 40 min (Fig. 1). To assess the involvement of ADR or OPR in EGCG-induced cardioprotection, ADR and OPR antagonists were perfused via 2nd port 10 min before EGCG perfusion. The concentrations of all chemicals were based on our and other previous studies on isolated working rat hearts that had no effect on infarct size in hearts subjected to ischemia and reperfusion [5,7-11].
At the end of each experiment, the LCA perfusion circuit was precluded, and diluted fluorescent polymer microspheres (Duke Scientific Corp., Palo Alto, MA, USA) were infused to demarcate the area at risk (AR). The hearts were cut into 2-mm thick transverse slices using a rat heart slice matrix (Zivic Instruments, Pittsburgh, PA, USA). The slices were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich Chemical, St. Louis, MO, USA) in sodium phosphate buffer (pH = 7.4) at 37℃ for 20 min and subsequently immersed in 10% formalin to enhance the contrast. The left ventricle (LV) was removed from the remaining tissue. The myocardial AR in the LV was identified by illuminating with UV light. The necrotic area (AN, unstained with TTC) and AR (nonfluorescent under UV light) were traced on a clear acetate transparent sheet (Fig. 2B and 2C) and quantified with UTHSCSA Image Tool, version 3.0 (Department of Dental Diagnostic Science at The University of Texas Health Science Center, San Antonio, Texas, USA). The areas were converted into volumes by multiplying them by slice thickness. The AN volume was expressed as a percentage of the AR volume. All morphometric measurements were performed in a blinded fashion by a separate technician.
Data are presented as means ± SEM. Data analysis was performed with a personal computer statistical software package (SPSS for windows, Release 17.0; SPSS Inc, Chicago, IL, USA). Data were analyzed using one-way analysis of variance (ANOVA) with Tukey's HSD post-hoc testing. Null hypotheses of no difference were rejected if P values were less than 0.05.
A total of 96 rat hearts were used for infarct measurement experiment. Four rats were excluded during the stabilization period because of a CF > 18 ml/min or < 8 ml/min (2), LVDP < 80 mmHg (1), or HR < 250 beats/min (1). A further two hearts were excluded due to irreversible post-ventricular fibrillation pump failure (1 in control and 1 in EGCG + ZM241385). Therefore, we report the data for 90 successfully completed infarct experiments (each group n = 9).
There were no significant group differences in body weight, heart weight, heart to body weight ratio, LV volume, AR volume and AR to LV ratio (Table 1). Ten µM of EGCG targeting reperfusion significantly reduced infarct size over risk area from 33.5 ± 4.1% to 14.4 ± 1.1% (P < 0.001 vs. CON) (Fig. 3). Ten µM of the nonspecific OPR antagonist naloxone (14.3 ± 1.3%, P < 0.001 vs. CON) could not block the infarct-limitation effect by EGCG. However, 1 µM of the nonspecific ADR antagonist 8-SPT (27.1 ± 1.9%, P > 0.05 vs. CON) blocked the anti-infarct effect by EGCG. Naloxone and 8-SPT itself did not alter infarct size (30.9 ± 4.5% for NAL and 29.6 ± 3.2% for 8-SPT, P > 0.05 vs. CON).
All four ADR antagonists had a tendency to attenuate the infarct-sparing effect by EGCG. The infarct reducing effect of EGCG was significantly reversed by 200 nM of the A1 ADR antagonist DPCPX (25.9 ± 1.9%, P < 0.01) and 15 nM of the A2B ADR antagonist MRS1706 (29.3 ± 1.7%, P < 0.01) but not by 10 µM of the A2A ADR antagonist ZM241385 (23.9 ± 1.9%) and 100 nM of the A3 ADR antagonist MRS1334 (24.1 ± 1.8%) (Fig. 4).
In the present study, EGCG targeting reperfusion effectively reduced infarct size after myocardial ischemia and reperfusion. Interestingly, the infarct reducing effect by EGCG was totally blocked by a nonspecific ADR antagonist 8-SPT but not by a nonspecific OPR antagonist naloxone, implying the involvement of ADR in EGCG-induced cardioprotection. We further tested the involvement of subtypes of ADR in the infarct limitation effect by EGCG with four different ADR subtypes (A1, A2A, A2B, and A3) antagonists. A1 and A2B ADR subtype antagonists attenuated the infarct-sparing effect by EGCG. Our data suggest, for the first time, that EGCG-induced cardioprotection may attenuate myocardial ischemia-reperfusion injury, at least in part, through ADR activation.
Meanwhile, our present data clearly suggest that there is a functional coupling between EGCG and ADR in mediating EGCG-induced cardioprotection in the isolated rat heart, although the exact mechanism of this interaction between EGCG treatment and cardiac ADR is not known. One possible mechanism is that EGCG may further increase myocardial levels of adenosine. Adenosine is produced primarily through the metabolism of adenosine triphosphate (ATP) and its level increases during stressful situations when ATP utilization increases, such as in our myocardial ischemia reperfusion injury model. Therefore, it may be possible that EGCG further increase the release of adenosine. However, there is scant information about the adenosine level change by EGCG treatment in the heart. Navarro-Peran et al. [12] demonstrated that EGCG produces a significant increase in a specific ADR in colon cancer cells and adenosine can modulate different signaling pathways by binding to its specific receptors. In their study, A3 ADR antibody expression was significantly increased after EGCG treatment in cytosolic fractions of Caco-2 cells. Therefore, the change in adenosine level by EGCG treatment in the myocardial ischemia-reperfusion model should be determined in the future. Meanwhile, cardioprotection does not necessarily correlate with increased adenosine production. Thus, adenosine concentration is not crucial to the beneficial effects of ischemia-reperfusion of the injured rat heart [13]. In this regard, another possible mechanism might involve endogenous activation of ADR itself without increase in adenosine level.
ADR is a GPCR and there are four subtypes, A1, A2A, A2B and A3. The activation of these ADR subtypes has been shown to be cardioprotective [14-16]. During the past two decades, numerous mechanisms of adenosine-mediated cardioprotection have been proposed. ADR activation has been suggested to reduce cell death through the mitochondrial KATP (mKATP) channel as well as protein kinase C and mitogen-activated protein kinase (MAPK) signaling [17,18]. Meanwhile, we previously reported the role of mKATP channel in EGCG-induced cardioprotection in isolated rat hearts [2]. The infarct limitation effect by EGCG was totally blocked by the non-selective KATP channel blocker glibenclamide and the selective mKATP channel blocker 5-hydroxydecanoate, implying that KATP channels, especially mKATP channels, play a crucial role in the cardioprotection by EGCG. Taken together, it is highly plausible that EGCG may provide cardioprotection via mKATP channel activation by ADR activation. However, the involvement of MAPK signaling in EGCG-induced cardioprotection is not clear. Previous studies in this laboratory have shown that that EGCG treatment did not increase the phosphorylation of ERK and Akt compared to untreated control hearts (data not shown). Therefore, it is not likely that ADR activation by EGCG involves MAPK downstream signaling.
In the present study, we perfused EGCG targeting early reperfusion phase. Interestingly, it has been proposed that activation of both A1 and A3 ADR is cardioprotective when triggered ischemic period. For example, intracoronary injection of A1 ADR agonist N6-1-(phenyl-2R-isopropyl)adenosine reduced infarct size as effective as ischemic preconditioning in isolated rabbit heart [19]. In contrast, it has been demonstrated that the cardioprotection by activation of A2 ADR is beneficial during reperfusion [20,21]. Potent A2B ADR agonist NECA infused from 5 min reperfusion mimicked ischemic postconditioning's effect on infarct size in in situ rabbit hearts [22]. In this study, EGCG-induced infarct limitation was blocked by A1 and A2B ADR. This suggests ADR may be involved in the EGCG treatment targeting both ischemia and reperfusion.
In conclusion, the infarct reducing effect of EGCG appears to involve activation of ADR, especially A1 and A2B ADR, but not OPR in isolated rat heart. Further studies in an ADR knockout mouse heart model and ADR level measurement by Western blot analysis should be undertaken in the future.
References
1. Song DK, Jang Y, Kim JH, Chun KJ, Lee D, Xu Z. Polyphenol (-)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial KATP channel activation in isolated rat hearts. J Korean Med Sci. 2010; 25:380–386. PMID: 20191036.
2. Kim CJ, Kim JM, Lee SR, Jang YH, Kim JH, Chun KJ. Polyphenol (-)-epigallocatechin gallate targeting myocardial reperfusion limits infarct size and improves cardiac function. Korean J Anesthesiol. 2010; 58:169–175. PMID: 20498796.

3. Hansen JL, Theilade J, Aplin M, Sheikh SP. Role of G-protein-coupled receptor kinase 2 in the heart--do regulatory mechanisms open novel therapeutic perspectives? Trends Cardiovasc Med. 2006; 16:169–177. PMID: 16781951.

4. McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther. 2012; 17:21–33. PMID: 21335481.
5. Chen Z, Li T, Zhang B. Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res. 2008; 145:287–294. PMID: 18155248.

6. Chun KJ, Park YH, Kim JS, Jang Y, Kim JH, Kim J, et al. Comparison of 5 different remifentanil strategies against myocardial ischemia-reperfusion injury. J Cardiothorac Vasc Anesth. 2011; 25:926–930. PMID: 21514843.

7. Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. Inhibiting protease-activated receptor 4 limits myocardial ischemia/reperfusion injury in rat hearts by unmasking adenosine signaling. J Pharmacol Exp Ther. 2008; 324:1045–1054. PMID: 18055876.

8. Ebrahimi S, Faghihi M, Keshavarz M, Kadkhodaee M, Mirershadi F, Asadi B. Anti-infarct effect of magnesium is not mediated by adenosine A1 receptors in rat globally ischaemic isolated hearts. Clin Exp Pharmacol Physiol. 2004; 31:868–872. PMID: 15659051.

9. Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, et al. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009; 47:684–690. PMID: 19695259.
10. Monahan TS, Sawmiller DR, Fenton RA, Dobson JG Jr. Adenosine A(2a)-receptor activation increases contractility in isolated perfused hearts. Am J Physiol Heart Circ Physiol. 2000; 279:H1472–H1481. PMID: 11009431.

11. Park SS, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-iodobenzyl)-adenosine-5'-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3 beta. J Pharmacol Exp Ther. 2006; 318:124–131. PMID: 16611852.
12. Navarro-Perán E, Cabezas-Herrera J, Campo LS, Rodríguez-López JN. Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. Int J Biochem Cell Biol. 2007; 39:2215–2225. PMID: 17683969.
13. Vasara E, Katharou I, Lazou A. Myocardial adenosine does not correlate with the protection mediated by ischaemic or pharmacological preconditioning in rat heart. Clin Exp Pharmacol Physiol. 2003; 30:350–356. PMID: 12859425.

14. Smits GJ, McVey M, Cox BF, Perrone MH, Clark KL. Cardioprotective effects of the novel adenosine A1/A2 receptor agonist AMP 579 in a porcine model of myocardial infarction. J Pharmacol Exp Ther. 1998; 286:611–618. PMID: 9694911.
15. Safran N, Shneyvays V, Balas N, Jacobson KA, Nawrath H, Shainberg A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol Cell Biochem. 2001; 217:143–152. PMID: 11269659.
16. Maddock HL, Mocanu MM, Yellon DM. Adenosine A(3) receptor activation protects the myocardium from reperfusion/reoxygenation injury. Am J Physiol Heart Circ Physiol. 2002; 283:H1307–H1313. PMID: 12234780.
17. Liang BT. Direct preconditioning of cardiac ventricular myocytes via adenosine A1 receptor and KATP channel. Am J Physiol. 1996; 271:H1769–H1777. PMID: 8945890.
18. Ballard-Croft C, Kristo G, Yoshimura Y, Reid E, Keith BJ, Mentzer RM Jr, et al. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. Am J Physiol Heart Circ Physiol. 2005; 288:H1359–H1366. PMID: 15539417.

19. Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991; 84:350–356. PMID: 2060105.

20. Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, et al. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)-adenosine-5-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997; 80:800–809. PMID: 9168782.
21. Zhao ZQ, McGee S, Nakanishi K, Toombs CF, Johnston WE, Ashar MS, et al. Receptor-mediated cardioprotective effects of endogenous adenosine are exerted primarily during reperfusion after coronary occlusion in the rabbit. Circulation. 1993; 88:709–719. PMID: 8339431.

22. Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006; 70:308–314. PMID: 16545350.

Fig. 1
Experimental protocols. Hearts were subjected to 30 min of regional ischemia and 2 h of reperfusion. Polyphenol (-)-epigallocatechin gallate (EGCG) was perfused from 5 min before reperfusion to 30 min after reperfusion. Adenosine or opioid receptor antagonists were pre-treated 10 min before EGCG perfusion.
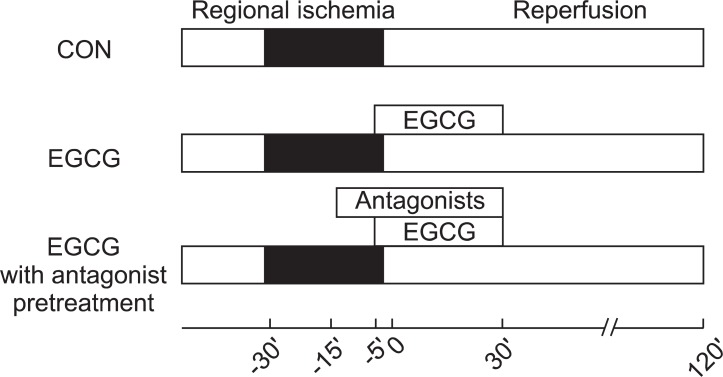
Fig. 2
(A) Regional ischemia was induced by pulling the snare at the level of the proximal left coronary artery, (B) The area at risk of left ventricle slice was identified by UV light illumination as the tissue without fluorescence. (C) The area of necrosis was identified by unstained area by TTC (closed circle) in area at risk.
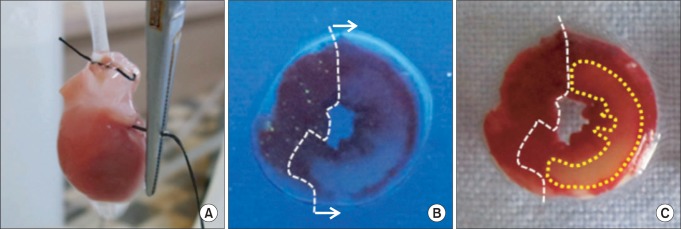
Fig. 3
(A) Representative sequential left ventricle (LV) slices from each group showing area of necrosis (pale area) with TTC staining. (B) % of infarct area (AN) over area at risk (AR). All data are expressed as means ± SEM. CON: untreated control hearts, EGCG: polyphenol (-)-epigallocatechin gallate, NAL: nonspecific opioid receptor antagonist naloxone, SPT: nonspecific adenosine receptor antagonist 8-(p-sulfophenyl)theophylline hydrate. *P < 0.05 vs. CON, †P < 0.05 vs. EGCG.
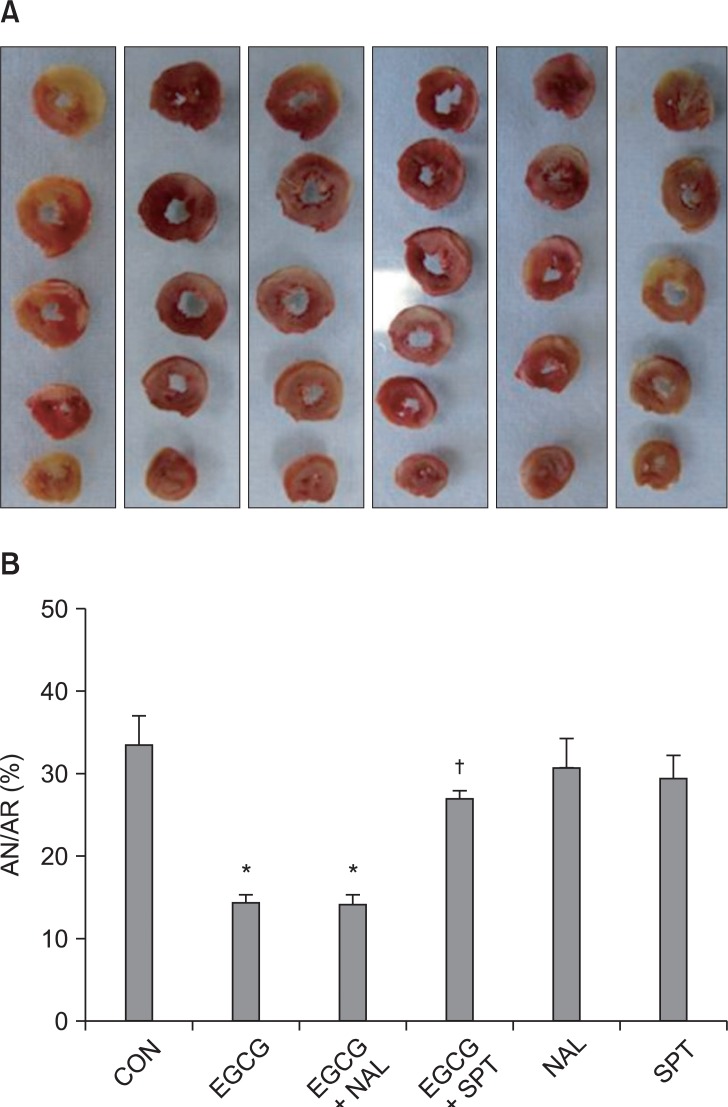
Fig. 4
(A) Representative sequential left ventricle (LV) slices from each group showing area of necrosis (pale area) with TTC staining after adenosine receptor (ADR) antagonist pretreatment in EGCG treat hearts. (B) % of infarct area (AN) over area at risk (AR). All data are expressed as means ± SEM. CON: untreated control hearts, EGCG: polyphenol (-)-epigallocatechin gallate, DPCPX: A1 ADR antagonist, ZM241385: A2a ADR antagonist, MRS1706: A2b ADR antagonist, MRS1334: A3 ADR antagonist. *P < 0.05 vs. CON, †P < 0.05 vs. EGCG.
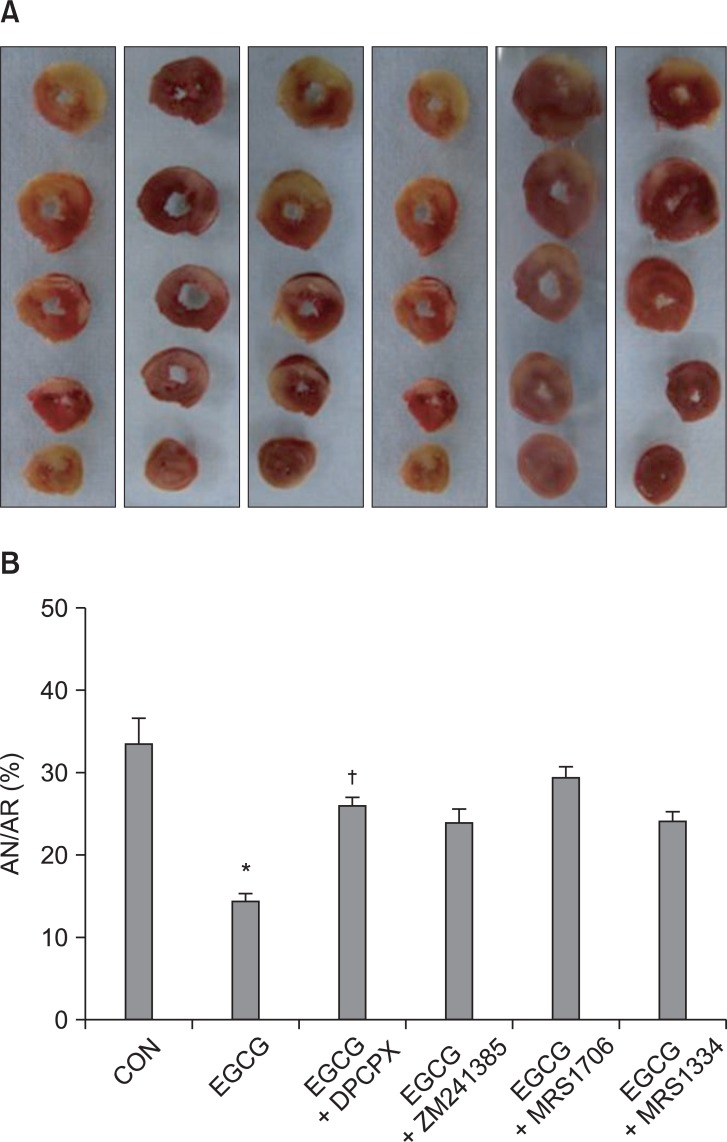
Table 1
Morphometric Data (each group n = 9)
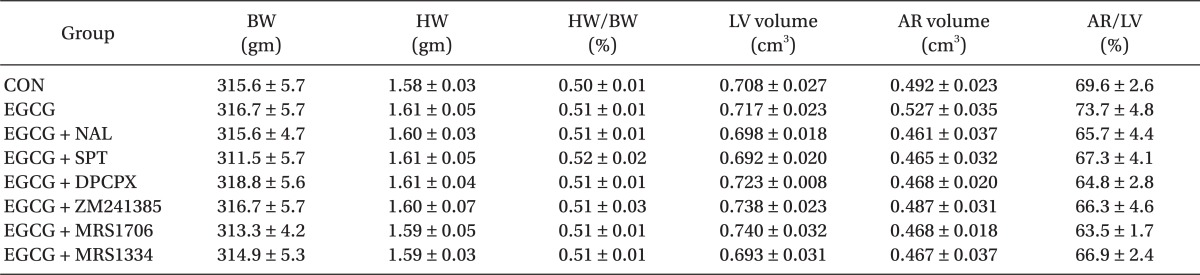
Values are means ± SEM. CON: untreated control heart, EGCG: polyphenol (-)-epigallocatechin gallate, NAL: nonspecific opioid receptor antagonist naloxone, SPT: nonspecific adenosine receptor (ADR) antagonist 8-(p-sulfophenyl)theophylline hydrate, DPCPX: A1 ADR antagonist, ZM241385: A2a ADR antagonist, MRS1706: A2b ADR antagonist, MRS1334: A3 ADR antagonist, BW: body weight, HW: heart weight, LV: left ventricle, AR: area at risk. There were no significant differences among groups.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download