Abstract
Background
Cerebral hypotension and desaturation can occur during shoulder surgery in the seated position. We evaluated the correlation of cerebral oxygen saturation (rSO2) using near infra-red spectroscopy (NIRS) and mean arterial pressures (MAP) (at the levels of the brain and heart).
Methods
Fifty patients, scheduled for the arthroscopic shoulder surgery in the seated position, were enrolled to monitor the rSO2, bispectral Index (BIS), and MAPs at the levels of the brain and heart. The values of each parameter were collected at 5 min after intubation, immediately after placing the patient in the sitting position, 5 min after the patient was seated, immediately after the surgical incision, and every 30 min after incision.
Arthroscopic shoulder surgery on patients in a seated position has recently become common. A comparison of the lateral supine position to the sitting position reveals that the seated position has some surgical advantages including: an excellent anatomical position, a decreased need to use arm traction, a brachial plexus injury risk reduction, a good surgical field, and a positive outcome [1,2]. Also, the surgical field is placed at a higher level than heart and surgeons can get a good surgical view in the sitting position.
Four cases of catastrophic central nervous system injury have been reported shoulder surgery in the upright position. All were performed under general anesthesia, and attributed to brain hypoperfusion due to postural hypotension. The authors attributed the complications to intraoperative reductions in cerebral perfusion pressure (CPP) [3]. There was also a report of perioperative visual loss associated with arthroscopic shoulder surgery although the etiology in that case is less clear [4]. Reported cases of postoperative neurological deficit have highlighted the risk of cerebral and spinal cord ischemia resulting from postural hypotension and cerebral hypoperfusion. The safe lower limit of hypotension is uncertain so far [5]. As well, little is known about the cerebrovascular effects of relative hypotension in shoulder arthroscopic patients anesthetized in the sitting position.
The sitting position for neurosurgery involving the cervicodorsal spine and the posterior and lateral cranial fossae was the most popular in the 1960s and 1970s, because gravity could aid drainage of blood and facilitate surgical procedure [6]. Although this position provides excellent surgical access and can be relatively safe, malpractice liability claims and serious complications, such as hemodynamic instability, pneumocephalus, and quadriplegia, induced the decline of neurosurgeries in the sitting position. To monitor adequate cerebral perfusion of anesthetized patients in the sitting position in neurosurgery, the placement of a pressure transducer at the level of the external auditory canal has been recommended [7].
Severe neurologic pathology, such as stroke, can be a major cause of morbidity and occur in up to 6% after cardiac surgery [8]. Murkin et al. [9] showed a consistent clinical benefit to monitoring and managing cerebral oxygen saturation (rSO2) using near infra-red spectroscopy (NIRS) during coronary artery bypass surgery. Hayashida et al. [10] reported that the NIRS-BIS (bispectral index) combination can be a convenient monitor of cerebral ischemia during pediatric cardiac surgery.
This study aimed to investigate the correlation of mean arterial pressure (at the levels of the brain and heart), and rSO2 using NIRS in patients placed in the sitting position for shoulder surgery under general anesthesia.
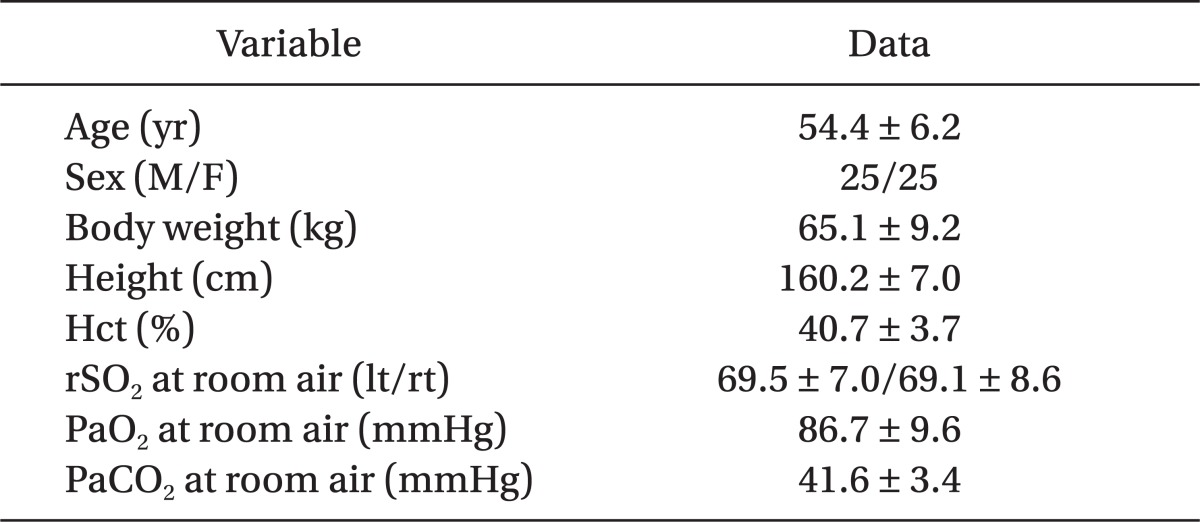
The study was approved by the Institutional Review Board of Ulsan University Hospital. Fifty patients, between 20 to 65 years of age and the American Society of Anesthesiologists (ASA) physical status classification I or II, were enrolled for this study. They underwent elective arthroscopic shoulder surgery in a seated position under general anesthesia between May 2009 and Apr 2010. We obtained written informed consents from all patients after a full explanation of this study. Patients with a body mass index (BMI) greater than 30 kg/m2, anemia (Hb lesser than 10.0 g/dl), and significant respiratory, cardiovascular, metabolic, endocrinologic, or central nervous system disease were excluded from the study group. Patients' characteristics are shown in Table 1.
Before the induction of anesthesia, standard monitors, such as electrocardiogram, peripheral oxygen saturation (SpO2), and non-invasive arterial pressure, were attached to all patients and recorded at 5 min interval to the end of operation. After a modified Allen's test and infilteration of 2% lidocaine, the radial artery on the non-operative side was catheterized for invasive monitoring of arterial pressure. Two arterial pressure transducers were connected to this artery. One of those sensors was placed at the level of the external auditory meatus and the other sensor was placed at the level of heart. Also, we monitored the bispectral index (BIS) and rSO2 values, using a BIS monitor (A-2000, Aspect medical Systems, Natick, MA, USA) with a BIS™ sensor and a cerebral oximeter (INVOS 5100, Somanetics Corp., Troy, MI, USA) with an adult sensor (Somasensor®, Somanetics Corp., Troy, MI, USA) using NIRS, respectively. The baseline mean arterial pressure (MAP), heart rate (HR), SpO2, BIS, and left and right rSO2 values were recorded while the patient's condition was stabled over 5 minutes before induction. Subsequently, the MAP at the level of upper arm (heart) and external auditory meatus, BIS, EtCO2, HR, SpO2, and left and right rSO2 were recorded at 5 mins after intubation, immediately after the patient was seated, 5 mins after patient was seated, immediately following the surgical incision, and every 30 min after incision. From the radial artery, arterial blood gas analysis (ABGA) was done to check PaCO2 and PaO2 as a baseline before O2 administration by face mask. ABGA was intermittently performed to compare the difference between PaCO2 and EtCO2 through the operation and ventilator setting was controlled to keep PaCO2 40 mmHg.
All the patients were not premedicated. After recording every parameter value as a baseline and sampling of blood from a radial artery for arterial blood gas analysis of spontaneous respiration, 100% oxygen was administered to all patients from the circuit of anesthetic machine. Anesthesia was induced using pentothal sodium 4-5 mg/kg IV and remifentanil 0.1-0.15 µg/kg/min IV. After the patient was unable to respond to verbal commands and eyelid reflex was absent, lidocaine 40-60 mg was injected intravenously followed by rocuronium 0.8-1.0 mg/kg to facilitate endotracheal intubation. Endotracheal intubation was performed, using 7.0-7.5 mm (internal diameter) reinforced tracheal tube, 90-120 seconds after injection of rocuronium in all patients. Cuff pressure was maintained at 15-25 cmH2O with a hand pressure gauge (Hi-Lo™ Hand Pressure Gauge, VBM Medizintrchnik GmbH, Germany) throughout the procedure. Anesthesia was maintained with sevoflurane, 50% O2, and remifentanil, which were titrated to maintain the MAP and HR within 10-20% of preinduction values and to keep BIS values between 40 and 60. But the sevoflurane and remifentanil levels were kept at least more than 1.0 vol% and 0.02 µg/kg/min, respectively. Hypotension, MAP of < 60 mmHg at the level of external auditory meatus, was treated with ephedrine 5 mg IV when the heart rate (HR) was < 60 bpm, or with phenylephrine 25 µg IV when HR was > 60 bpm. Mechanical ventilation was maintained with a tidal volume of 8 ml/kg, and ventilator frequency was adjusted to maintain PaCO2 40 mmHg. Body temperature was maintained at 36-37℃.
At the end of surgery, mechanical ventilation was discontinued and assisted manual ventilation was provided with 100% oxygen. Extubation was performed when patients were able to open their eyes and squeeze both hands on command.
All data are expressed as mean ± standard deviation (SD). Data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The correlation between MAPs at the level of heart and brain and left and right rSO2 was analyzed with the Pearson correlation test. Repeated measures analysis of variance (ANOVA) was adopted to analyze the change over time of each parameter, such as HR, MAP, left and right rSO2, and BIS. A P value of < 0.05 was considered statistically significant.
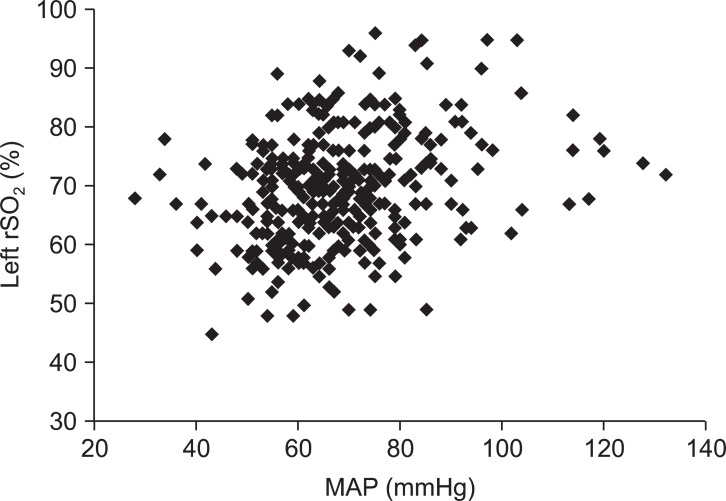
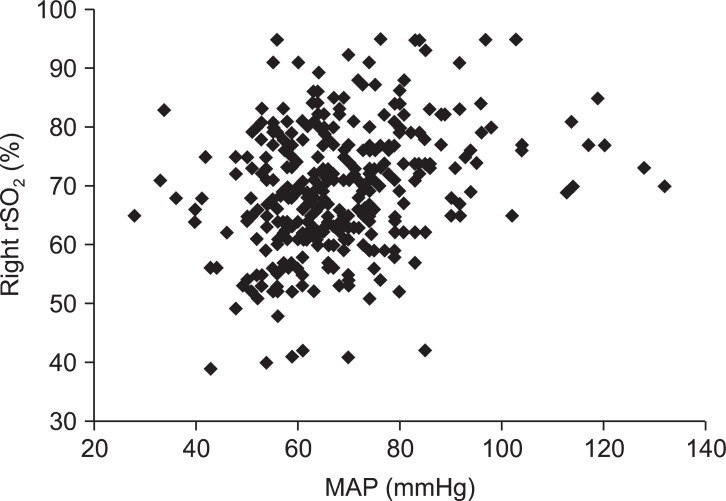
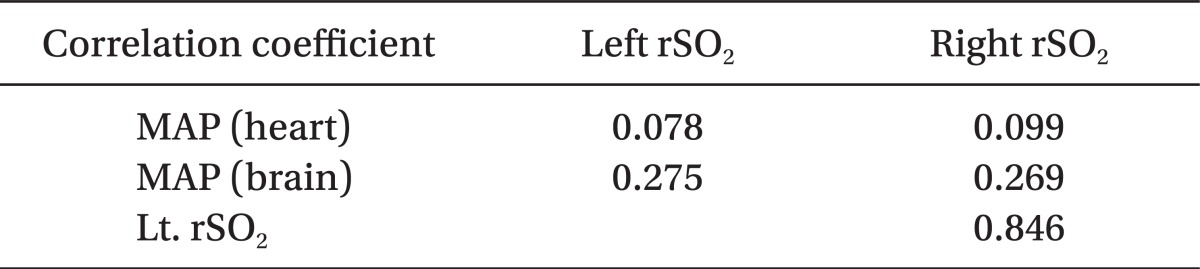
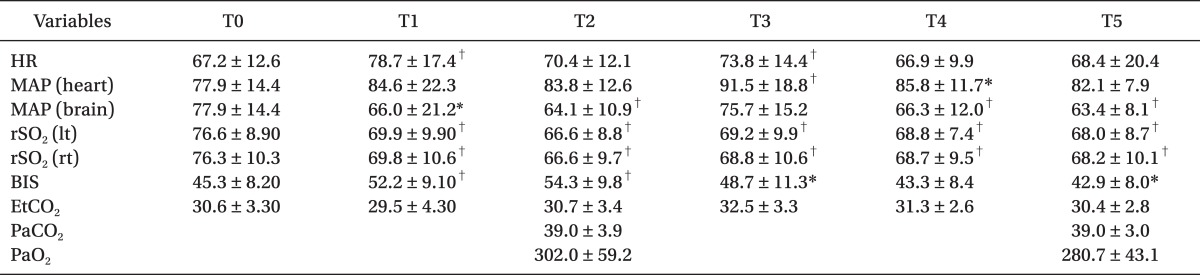
There were statistically significant correlations between MAP at the level of external auditory meatus and the rSO2 on the left and right sides (P < 0.01) (Fig. 1 and 2, Table 2). But, the correlations between MAP at the level of heart and both rSO2 were not statistically significant (P > 0.05, Table 2).
Left and right rSO2 after a posture change from supine to sitting position were significantly decreased (Table 3). And MAP at the brain level was significantly decreased compared to the baseline value measured at the supine position (Table 3).
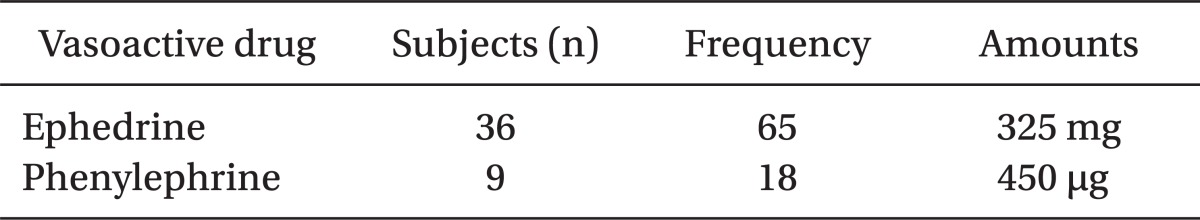
We had a clinical target to keep MAP at the level of brain 60 mmHg or more and we used a vasoconstrictor, such as ephedrine and phenylephrine. Used frequency and the total amount of ephedrine were 65 times and 325 mg to the 36 patients and those of phenylephrine were 18 times and 450 µg to the 9 patients. Eight patients had received ephedrine and phenylephrine together to keep MAP higher than 60 mmHg (Table 4). Vasoconstrictors were injected in 37 of 50 patients (74%).
There were no significant correlations statistically between BIS and MAP at the levels of the heart and brain at the sitting position. BIS was significantly higher immediately after sitting position, compared to baseline value (Table 3).
In the present study, rSO2 and MAP significantly decreased after a postural change from supine to sitting position for arthroscopic shoulder surgery. Moreover, MAP measured in the level of external auditory meatus, not MAP of heart level, was significantly correlated with left or right rSO2 measured by NIRS in the seated patients for shoulder surgery.
The advantage of the sitting position for arthroscopic shoulder surgery includes placement of the anatomy in the standard upright position without distortion of the intra-articular anatomy, reduced use of arm traction, and less bleeding in the upright position [1,2]. Historically, the sitting position was first introduced into clinical surgery in the neurosurgical practice in 1930 [7]. But, serious complications related to this position include hemodynamic instability, venous air embolism with the possibility of paradoxical air embolism, pneumocephalus, quadriplegia, and compressive peripheral neuropathy [11]. Posterior fossa craniotomies performed in the sitting position declined from 110 to less than 50 over 4-year period from 1981 to 1984 at the Mayo Clinic, USA [12].
There are various neurologic complications from postoperative cognitive dysfunction (POCD) to stroke following surgery under general anesthesia. Especially, after cardiac surgery, there were frequent occurrences of POCD, for which cerebral microembolism and hypoperfusion have been proposed to be the major mechanisms [13,14]. Decrease in rSO2 measured by NIRS oximeter suggests a decrease in cerebral oxygen delivery and the cerebral oxygen desaturation is shown to be associated with early postoperative neuropsychological dysfunction after cardiac surgery [15]. The BIS also allows for detection of cerebral ischemia, particularly if it is used along with NIRS to indicate cerebral tissue oxygenation [16]. Thus Hayashida et al. [10] suggested the BIS-NIRS combination can be a convenient cerebral ischemic/anesthetic monitor during cardiac surgery in children.
After all, in the shoulder surgery, Pohl and Cullen [3] reported 4 cases of patients placed in the beach-chair position that resulted in death for 1 patient and severe brain damage for 3 patients. Reduced cerebral blood flow due to poorly compensated hypotension in the anesthetized state was thought as the main cause of neurologic sequelae in the sitting position for shoulder surgery, Visual loss and ophthalmoplegia after arthroscopic shoulder surgery in a 90 degree upright sitting position have also been described [4]. The different point to consider carefully in this case was that blood pressure monitoring was performed with a noninvasive cuff placed above the ankle on the right leg and systolic blood pressure was maintained at 100 mmHg throughout the 98 min procedure in this case. This gave us some hint that the level of measurements of blood pressure in the sitting position should be considered as an important factor to compare.
Porter et al. [7] defined CPP as the difference between mean cerebral arterial pressure and mean central venous pressure. They insisted that positioning the arterial pressure transducer at the level of the mid-cerebrum allows estimation of CPP of patients at the sitting position in neurosurgery. A MAP of 60 mmHg has been recommended by some investigators as the lower limit of deliberate hypotension [17]. Others suggest allowable systolic blood pressure be 20-30% below baseline (to 80-90 mmHg in normal patients) during controlled hypotension [18]. Soeding et al. [19] recommended that MAP be maintained at 70 mmHg at the tragus level and CPP be estimated to be 60 mmHg or above to prevent cerebral hypoperfusion. But, there is no known safe lower limit of blood pressure to protect brain so far [5]. In the supine position, blood pressure (BP) measured in the arm and pressure delivered to the brain is essentially same. However, if the patient is upright in the beach chair position, the BP will be lower at the level of brain than the heart or arm. In addition, surgeons sometimes ask for deliberate hypotension to decrease intra-articular bleeding and achieve better visualization during shoulder arthroscopy.
Specifically, we have shown that NIRS rSO2 is significantly correlated to brain MAP, not MAP from the arm. NIRS rSO2 decreased around 15% from baseline (78 → 66) but did not decrease to the level of cerebral desaturation, defined as a 20% reduction in rSO2 from baseline [20]. Actually, whenever brain MAP decreased to less than 60 mmHg, we used vasopressor, ephedrine or phenylephrine, to prevent cerebral hypoperfusion and minimize brain ischemia, as is routine in our practice.
We have some limits. First, there was no intraoperative ICP monitoring. It could be helpful to calculate CPP, if we had been able to do ICP monitoring. Second, if we could use transcranial doppler (TCD), it could be helpful to compare the change of cerebral blood flow according to position change. The third limit was we could not evaluate the preoperative and postoperative cognitive function. Actually we compared preliminarily preoperative and postoperative Mini-Mental State Examination (MMSE) of 12 patients in the beginning of this study; there were no differences of them and we could not keep checking MMSE of the other patients because of clinical difficulties.
In conclusion, this investigation suggests that rSO2 in patients placed in the sitting position for shoulder surgery under general anesthesia can be used to monitor and predict the development of cerebral hypoperfusion and ischemia. Even though it is not possible to specify an absolute rSO2 as the critical value below which cerebral ischemia may develop, monitoring rSO2 and MAP in the level of external auditory canal simultaneously is more appropriate to predict cerebral oxygen desaturation earlier and can help prevent cerebral ischemia. We think the further study is needed to clarify the allowable MAP in any levels.
Acknowledgments
This study was supported by a grant from Biomedical Research Center of Ulsan University Hospital. The authors would like to thank Professor Cheol In Yoo for the advice regarding statistical analysis of the data in this studies.
References
1. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O'Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988; 4:256–259. PMID: 3233114.

2. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning:lateral decubitus versus beach chair. Arthroscopy. 2009; 25:891–896. PMID: 19664509.
3. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005; 17:463–469. PMID: 16171668.

4. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003; 96:899–902. PMID: 12598282.

5. Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort suing automated data collection. Anesthesiology. 2007; 107:213–220. PMID: 17667564.
6. Gale T, Leslie K. Anesthesia for neurosurgery in the sitting position. J Clin Neurosci. 2004; 11:693–696. PMID: 15337126.
7. Porter JM, Pidgeon C, Cunningham AJ. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999; 82:117–128. PMID: 10325848.

8. Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996; 335:1857–1863. PMID: 8948560.
9. Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007; 104:51–58. PMID: 17179242.

10. Hayashida M, Kin N, Tomioka T, Orii R, Sekiyama H, Usui H, et al. Cerebral ischaemia during cardiac surgery in children detected by combined monitoring of BIS and near-infrared spectroscopy. Br J Anaesth. 2004; 92:662–669. PMID: 15033888.

11. Standefer M, Bay JW, Trusso R. The sitting position in neurosurgery: a retrospective analysis of 488 cases. Neurosurgery. 1984; 14:649–658. PMID: 6462398.
12. Black S, Ockert DB, Oliver WC Jr, Cucchiara RF. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988; 69:49–56. PMID: 3389566.

13. Barbut D, Yao FS, Hager DN, Kavanaugh P, Trifiletti RR, Gold JP. Comparison of transcranial doppeler ultrasonography and transesophageal echocardiography to monitor emboli during coronary artery bypass surgery. Stroke. 1996; 27:87–90. PMID: 8553410.
14. Moody DM, Bell MA, Challa VR, Johnston WE, Prough DS. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990; 28:477–486. PMID: 2252360.

15. Yao FS, Tseng CC, Ho CY, Levin SK, Illner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2004; 18:552–558. PMID: 15578464.

16. Hayashida M, Chinzei M, Komatsu K, Yamamoto H, Tamai H, Orii R, et al. Detection of cerebral hypoperfusion with bispectral index during paediatric cardiac surgery. Br J Anaesth. 2003; 90:694–698. PMID: 12697602.

17. Sollevi A. Hypotensive anesthesia and blood loss. Acta Anaesthesiol Scand Suppl. 1988; 89:39–43. PMID: 3067488.

18. Dutton RP. Controlled hypotension for spinal surgery. Eur Spine J. 2004; 13(Suppl 1):S66–S71. PMID: 15197633.

19. Soeding PF, Wang J, Hoy G, Jarman P, Phillips H, Marks P, et al. The effect of the sitting upright or 'beachchair' position on cerebral blood flow during anaesthesia for shoulder surgery. Anaesth Intensive Care. 2011; 39:440–448. PMID: 21675064.

20. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000; 93:964–970. PMID: 11020747.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download