1. Welborn LG, Hannallah RS, Norden JM, Ruttimann UE, Callan CM. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg. 1996; 83:917–920. PMID:
8895263.

2. Jones RM, Cashman JN, Eger EI 2nd, Damask MC, Johnson BH. Kinetics and Potency of desflurane (I-653) in volunteers. Anesth Analg. 1990; 70:3–7. PMID:
2297103.

3. Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007; 104:509–520. PMID:
17312200.

4. Davis PJ, Cohen IT, McGowan FX Jr, Latta K. Recovery Characteristics of desflurane versus halothane for maintenance of anesthesia in pediatric ambulatory patients. Anesthesiology. 1994; 80:298–302. PMID:
8311312.

5. Aono J, Mamiya K, Manabe M. Preoperative anxiety is associated with a high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol Scand. 1999; 43:542–544. PMID:
10342002.

6. Weldon BC, Bell M, Craddock T. The effect of caudal analgesia on emergence agitation in children after sevoflurane versus halothane anesthesia. Anesth Analg. 2004; 98:321–326. PMID:
14742362.

7. Cole JW, Murray DJ, McAllister JD, Hirshberg GE. Emergence behavior in chidren: defining the incidence of excitement and agitation following anaesthesia. Paediatr Anaesth. 2002; 12:442–447. PMID:
12060332.
8. Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology. 1997; 87:1298–1300. PMID:
9416712.

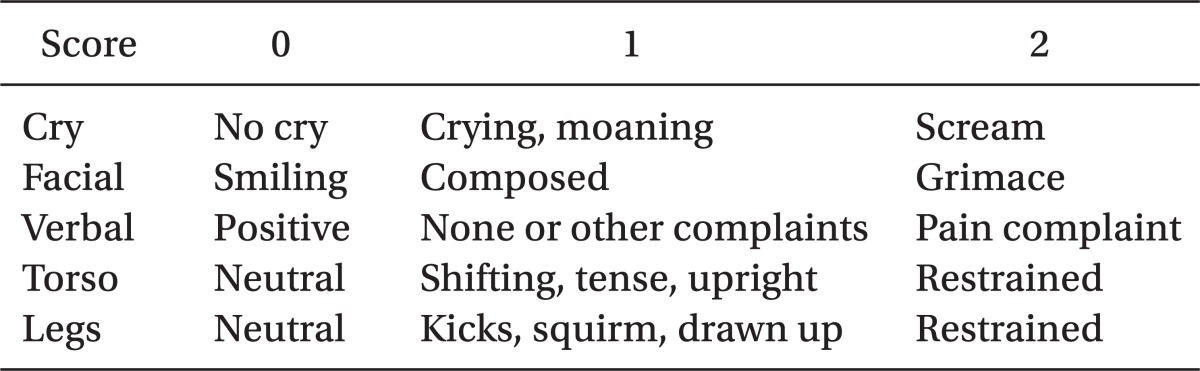
9. McGrath PJ, Johnson G, Goodman JT, Schillinger J, Dunn J, Chapman JA. Field HL, editor. CHEOPS: a behavioral scale for rating postoperative pain in children. Advances in pain research and therapy. 1985. 9th ed. New York: Raven Press;p. 395–402.
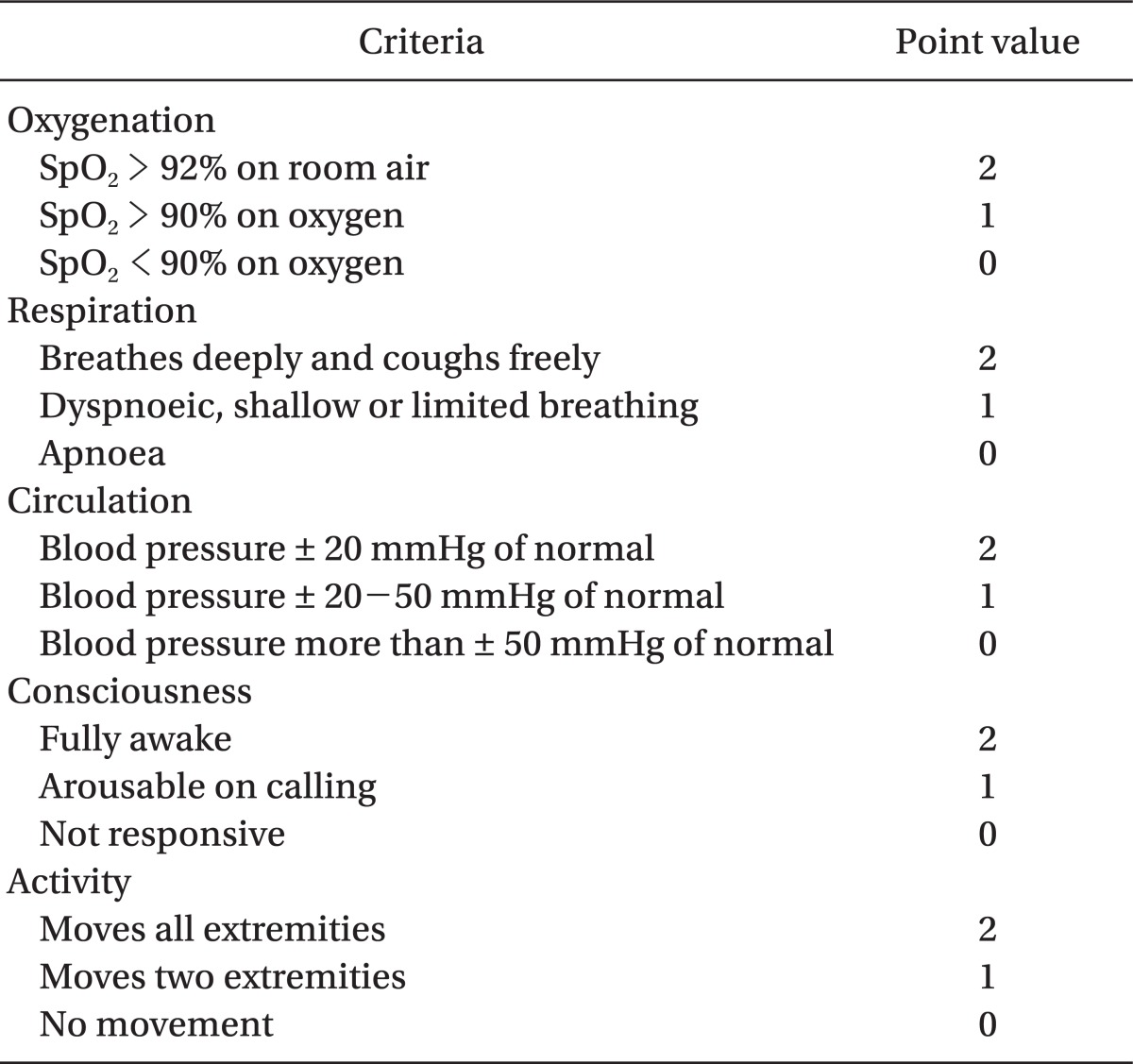
10. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995; 7:89–91. PMID:
7772368.

11. Grundmann U, Uth M, Eichner A, Wilheim W, Larsen R. Total intravenous anaesthesia with propofol and remifentanil in paediatric patients: a comparison with a desflurane-nitrous oxide inhalation anaesthesia. Acta Anaesthesiol Scand. 1998; 42:845–850. PMID:
9698963.

12. Valley RD, Freid EB, Bailey AG, Kopp VJ, Georges LS, Fletcher J, et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of desflurane and sevoflurane. Anesth Analg. 2003; 96:1320–1324. PMID:
12707126.

13. Uezono S, Goto T, Terui K, Ichinose F, Ishguro Y, Nakata Y. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth Analg. 2000; 91:563–566. PMID:
10960377.

14. Ebert TJ, Muzi M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers. A comparison with isoflurane. Anesthesiology. 1993; 79:444–453. PMID:
8363068.
15. Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003; 96:1625–1630. PMID:
12760985.

16. Abu-Shahwan I, Chowdary K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth. 2007; 17:846–850. PMID:
17683402.

17. Lim KJ, Choi KS, So KY, An TH. Comparison of incidences of emergence delirium from sevoflurane anesthesia in school and preschool children. Korean J Anesthesiol. 2003; 44:176–180.

18. Eckenhoff JE, Kneale DH, Dripps RD. The incidence and etiology of postanesthetic excitment. A clinical survey. Anesthesiology. 1961; 22:667–673. PMID:
13889092.
19. Aouad MT, Nasr VG. Emergence agitation in children: an update. Curr Opin Anaesthesiol. 2005; 18:614–619. PMID:
16534301.

20. Warncke T, Stubhaug A, Jorum E. Ketamine, an NMDA receptor antagonist, suppresses spatial and temporal properties of burn-induced secondary hyperalgesia in man: a double blind, cross-over comparison with morphine and placebo. Pain. 1997; 72:99–106. PMID:
9272793.
21. White PF, Way WL, Trevor AJ. Ketamine-its pharmacology and therapeutic uses. Anesthesiology. 1982; 56:119–136. PMID:
6892475.

22. Dich-Nielsen JO, Svendsen LB, Berthelsen P. Intramuscular low-dose ketamine versus pethidine for postoperative pain treatment after thoracic surgery. Acta Anaesthesiol Scand. 1992; 36:583–587. PMID:
1514347.

23. Kararmaz A, Kaya S, Turhanoglu S, Ozyilmaz MA. Oral ketamine premedication can prevent emergence agitation in chidren after desflurane anesthesia. Paediatr Anaesth. 2004; 14:477–482. PMID:
15153210.
24. Kawaraguchi Y, Miyamato Y, Fukumitsu K, Taniguchi A, Hirao O, Kitamura S, et al. The effect of ketamine on reducing postoperative agitation after sevoflurane anesthesia in pediatric strabismus surgery. Masui. 2002; 51:1343–1348. PMID:
12607270.
25. Kwak HJ, Kim JY, Kim JH, Kim YS, Park SY. The effect of ketamine and fentanyl on the incidence of emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy. Korean J Anesthesiol. 2005; 49:502–506.

26. Lee YS, Kim WY, Choi JH, Son JH, Kim JH, Park YC. The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean J Anesthesiol. 2010; 58:440–445. PMID:
20532051.






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download