Abstract
Diffuse alveolar hemorrhage (DAH) is an acute, life-threatening event. The blood-gas barrier must be very thin to allow gas exchange and is therefore subjected to high mechanical stresses when the capillary pressure rises. In general anesthesia, inhaled gases cause high mechanical stresses, and thus DAH occurs under certain conditions. We describe a case of inferred postoperative DAH. A 25-year-old man had an acute episode after undergoing a tonsillectomy for snoring. During surgery, no problems occurred and no marked bleeding was observed. After removal of the endotracheal tube, however, the patient had severe cough and hemoptysis. The patient was treated with an antihemorrhagic agent and antibiotics. He recovered after 1 week. Chronic snoring likely caused the alveolar damage in this patient and intubation led to DAH. The patient presented with a benign course that regressed spontaneously with medical intervention.
Diffuse alveolar hemorrhage (DAH) is a condition of bleeding from the alveolar capillaries into the alveoli. It is suspected in the presence of the typical triad of hemoptysis, anemia, and diffuse alveolar opacity, progressing to an acute condition, as shown on radiologic imaging [1-7]. The causes of massive hemoptysis in DAH are diverse. In most cases, hemoptysis is caused by collagenous disease or immunological conditions associated with vasculitis, and, although rare, it may also develop after vigorous exercise, after playing a wind instrument, or, in some cases, from barotrauma caused by anesthetic gas pressure during general anesthesia [4,5]. Here we describe a case of DAH that developed after tonsillectomy, and we review the literature regarding this condition.
A 25-year-old male patient visited our clinic for severe snoring. His height was 165 cm, his weight was 120 kg, and he had large palatine tonsils and severe snoring, which sometimes resulted in sleep apnea. His medical check-up, including a chest X-ray, revealed no other problems. In consideration of his sleep apnea symptoms, we planned a bilateral tonsillectomy and uvulopalatopharyngealplasty (UPPP) under general anesthesia. Intubation was difficult because of his short neck, but general anesthesia with Desfurane gas was stable during surgery. After the patient awoke from general anesthesia, we observed bloody sputum during endotracheal suction. Because we thought it was the result of aspiration during surgery, we removed the endotracheal tube.
The patient then developed a severe cough and hemoptysis. His vital signs were as follows: blood pressure, 120/70 mm Hg; temperature 38.4℃; heart rate, 106 beats/min; and respiration rate, 28 breaths/min. The SaO2 level was approximately 74% and the conjunctiva was pale. Heart sounds were normal, but wheezing was heard on chest auscultation. His white blood cell count was 29,190/mm3, hemoglobin (Hb) 13.7 g/dl, and platelet count 467,000/mm3. The erythrocyte sedimentation rate was 10 mg/dl and the C-reactive protein level was increased to 9.2 mg/dl; nevertheless, results of the fluorescent antinuclear antibody and antineutrophil cytoplasmic antibody (ANCA) tests were negative, and the levels of C3, C4, and rheumatoid factor were normal. After continuous hemoptysis for several hours, the patient's Hb level decreased rapidly to 6.8 g/dl and his blood SaO2 level decreased gradually.
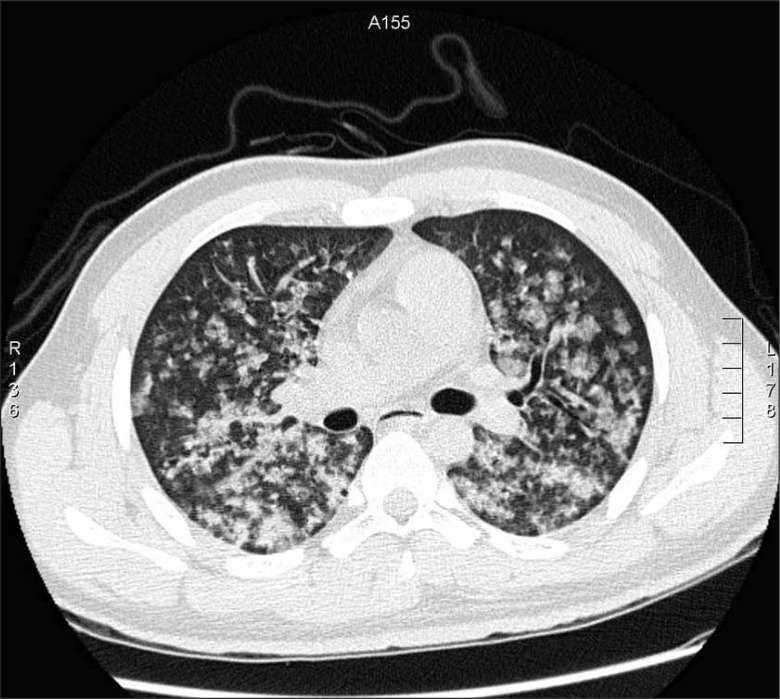
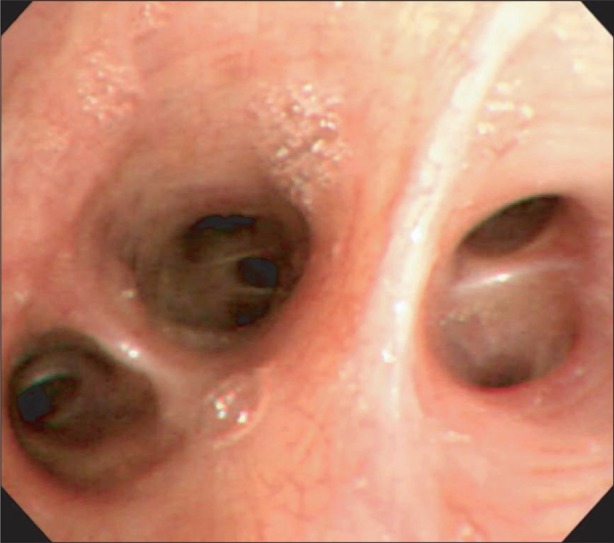
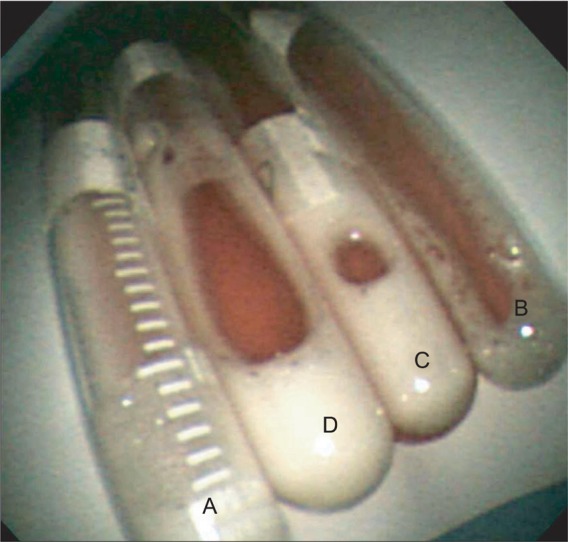
An immediate chest X-ray (Fig. 1A) and high-resolution computed tomography (Fig. 2) of the lower lobe in both lungs showed ground-glass opacity and induration with the appearance of alveolar infiltrates. The tonsillectomy site was clear, and a bronchoscopy was performed to find causative lesions within the trachea, but none were detected (Fig. 3). However, a bronchoalveolar lavage (BAL) of the right middle lobe bronchus was performed, and the bloody BAL fluid was observed to become redder (Fig. 4). The red blood cell count in the BAL fluid was elevated to 3,052/mm3, and on iron staining, hemosiderin-laden macrophages containing Hb were detected. Thus a definitive diagnosis of DAH was made, after which the patient was treated with an antihemorrhagic agent and antibiotics.
After the massive hemoptysis improved, on plain chest X-ray taken 1 week later, the ground-glass opacity and indurated lesion seen at admission disappeared completely (Fig. 1B). The patient was followed up for approximately 1 year, but no recurrence of disease could be detected.
DAH may induce acute respiratory distress syndrome, and in severe cases, it is quickly fatal. DAH can be classified broadly as an immunological or nonimmunological collagenous disease, or one that is associated with immunological causes, with vasculitis accounting for 65% of cases [6]. It can be induced by Goodpasture's syndrome (unrelated to tumors), microvasculitis associated with ANCA, collagenous vascular diseases, idiopathic pulmonary hemosiderosis, coagulation disorder, and diverse drugs and toxins. Although rare, it may develop after vigorous exercise, after playing a wind instrument, or, in some cases, from barotrauma caused by anesthetic gas pressure during general anesthesia [1-5].
In general, pulmonary hemorrhage can be diagnosed by hemoptysis, a decrease in Hb, and the presence of new infiltrations on plain chest X-ray. In addition, in the test for diffusing capacity of the lung for carbon monoxide, cases showing more than a 30% increase in normal values suggest DAH. Clinically, dyspnea and hypoxemia occur simultaneously in cases of pulmonary hemorrhage, but the initial chest X-ray may show diverse patterns; thus cases with accompanying anemia or hemoptysis should be particularly suspect. Pulmonary hemorrhage can be diagnosed by using chest computed tomography, chest magnetic resonance imaging, and bronchoscopy; nevertheless, cases that cannot be definitively diagnosed and cases with bloody lavage that gradually becomes redder (darker) can be diagnosed by examination of BAL fluid. In addition, diagnosis can be made by assessing macrophages containing hemosiderin from a biopsy sample taken during bronchoscopy. Nonetheless, a biopsy cannot be done in many cases because of the patient's condition; hence, pulmonary hemorrhage should be diagnosed by observing clinical features before performing various tests.
Similar to the treatment of hemorrhage in other conditions, that in DAH is treated by means of conventional methods such as administration of fresh frozen plasma, fibrin, and platelets [8].
In our patient, causes of DAH, such as immune disease, renal disease, vasculitis, and collagenous disease, could not be detected. Considering the timing of the appearance of DAH, and the fact that no traces of hemorrhage could be detected in the surgical area after tonsillectomy, we speculated that the DAH was due to barotrauma that developed after general anesthesia.
No problems occurred with general anesthesia during surgery. However, the patient was obese and had episodes of sleep apnea and snoring. Thus, severe chronic snoring may have damaged the alveolar wall, resulting in barotrauma during general anesthesia and causing DAH. We speculate that the anesthetic gas used for general anesthesia applied physical pressure to the alveoli and injured the very thin blood-gas barrier, resulting in the development of DAH.
Other cases of DAH have been reported that were thought to be caused by the development of barotrauma during marathon running, while playing a wind instrument strenuously, and during military training; nonetheless, the precise cause of DAH in these cases has not been elucidated [2,5].
In conclusion, we report a rare case of barotrauma caused by inhaled gas, which developed in our patient during tonsillectomy under general anesthesia. DAH was diagnosed by means of the BAL test and resolved with appropriate treatment.
References
1. Amstrong P, Wilson AG, Dee P, Hansell DM. Imaging of disease of the chest. 2000. 3rd ed. London: Mosby;p. 582–586.
2. Hopkins SR, Schoene RB, Henderson WR, Spragg RG, Martin TR, West JB. Intense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletes. Am J Respir Crit Care Med. 1997; 155:1090–1094. PMID: 9116992.

3. McKechnie JK, Leary WP, Noakes TD, Kallmeyer JC, MacSearraigh ET, Olivier LR. Acute pulmonary edema in two athletes during a 90-km running race. S Afr Med J. 1979; 56:261–265. PMID: 550480.
4. Ghio AJ, Chio C, Bassett M. Exercise-induced pulmonary hemorrhage after running a marathon. Lung. 2006; 184:331–333. PMID: 17086462.

5. Broccard AF, Liaudet L, Aubert JD, Schnyder P, Schaller MD. Negative pressure post-tracheal extubation alveolar hemorrhage. Anesth Analg. 2001; 92:273–275. PMID: 11133644.
6. Na JO, Kim SJ, Shim TS, Lim CM, Lee SD, Kim WS, et al. Twenty cases of diffuse alveolar hemorrhage : A single center retrospective study. Korean J Med. 2002; 62:258–267.
7. Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc. 2006; 3:48–57. PMID: 16493151.
8. Sane DC, Califf RM, Topol EJ, Stump DC, Mark DB, Greenberg CS. Bleeding during thrombolytic therapy for acute myocardial infaction: Mechanism and managements. Ann Intern Med. 1989; 111:1010–1022. PMID: 2688504.
Fig. 1
Serial chest X-ray. (A) Immediate postoperative chest x-ray, revealing that the lungs had bilateral patchy infiltrates. (B) Chest film showing resolution of the pulmonary infiltrates 7 days later.
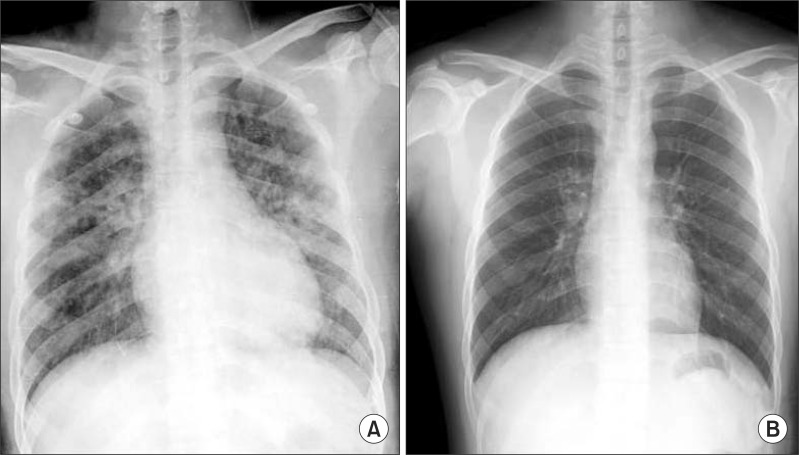
Fig. 2
Computed tomography image disclosing diffuse airspace disease involving all lobes, with bilateral ground-glass opacities.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download