Abstract
Background
Reactive oxygen species and inflammatory responses contribute to the development of neuropathic pain. Superoxide serves to mediate cell signaling processes and tissue injury during inflammation. We examined the effects of superoxide on the development and maintenance of mechanical allodynia, as well as its contribution to central sensitization in a superoxide-rich animal model of neuropathic pain.
Methods
Chronic post-ischemia pain (CPIP) was induced via the left hindpaw ischemia for 3 h, followed by reperfusion. Superoxide dismutase (4,000 U/kg, i.p.) was administered either 5 min before ischemia (BI), 5 min before reperfusion (BR), or 3 days after reperfusion (3AR). Withdrawal thresholds of the four paws were measured to assess the mechanical allodynia and the effects of circulating xanthine oxidase (XO)-mediated superoxide production. In addition, we measured the levels of N-methyl D-aspartate receptor subunit 1 phosphorylation (p-NR1) in the ipsilateral and contralateral spinal cord (L4-6), by Western blotting, to examine the superoxide-mediated central sensitization. Superoxide production was assessed by allopurinol-sensitive, XO-mediated lipid peroxidation of the spinal cord and gastrocnemius muscles.
Results
Withdrawal thresholds of forepaws did not vary across the 7 days of testing. In the hindpaws, both ipsilateral and contralateral mechanical allodynia was most attenuated in the BR group, followed by the BI and 3AR groups. The degree of NR1 activation was in contrast to the changes in the withdrawal thresholds.
Recent studies have proposed a role for reactive oxygen species (ROS) in neuropathic pain. Nitric oxide synthase inhibitors and ROS scavengers reduce the development of thermal hyperalgesia and mechanical allodynia in animal models of neuropathic pain [1,2]. In humans, patients suffering from complex regional pain syndrome type-I (CRPS-I) show more serum lipid peroxidation products and significantly elevated antioxidant parameters, such as salivary peroxidase and superoxide dismutase (SOD) [3]. Furthermore, CRPS symptoms are alleviated following the treatment with free radical scavengers [4].
Coderre et al. [5] developed an animal model of CRPS-I using a chronic post-ischemia pain (CPIP), which consists of ischemia/reperfusion (IR) injury of the rat hindpaw. Physical injury and symptoms arising from CPIP are comparable to those observed in CRPS-I. Chronic pain associated with CRPS-I following sprains, arthroscopic surgery, overly tight casting, and other edematous soft tissue injuries is associated with IR injury [5,6]. In CPIP rats, the ROS scavengers N-acetylcysteine and 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine-N-oxyl alleviate mechanical hyperalgesia [5,7]. Moreover, allopurinol, an inhibitor of xanthine oxidase (XO)-mediated superoxide production, as well as SOD and N-nitro-L-arginine methyl ester significantly alleviate mechanical and cold allodynia in CPIP rats [8]. However, the mechanisms by which ROS scavengers exert analgesic effects, and the specific ROS responsible for the development and maintenance of mechanical allodynia in CPIP rats, are unclear. One potential mechanism is superoxide-mediated central sensitization of the spinal cord. Mitochondrial ROS production is increased in both the superficial and deep dorsal horn in a model of neuropathic pain [9]. Since the primary type of ROS produced by mitochondria or XO is superoxide, it is likely that superoxide is important in central sensitization. Another relies on the circulating XO rather than the central sensitization. Superoxide produced by increased XO, following an IR injury, could directly interact with NO in the hindpaw, resulting in either decreased NO bioavailability and subsequent paw vessel vasoconstriction, or elevated peroxynitrite production and subsequent SOD nitration. Circulating XO has been shown to bind to and to be endocytosed into the vascular endothelium [10].
Different phases of neuropathic pain have not been clearly defined so far, and these phases may vary under different injury conditions. However, there are at least two phases in animal models: an early, development phase; and a late, maintenance phase [11]. In an animal model of neuropathic pain, the activation of signaling molecules, such as mitogen-activated protein kinases, show dynamic changes in different cell types at different times [12,13]. Furthermore, minocycline, a microglial inhibitor, attenuates neuropathic pain in the development phase, but not in the maintenance phase [14].
Therefore, based on the possible role of superoxide in neuropathic pain, the dynamic changes of cellular signaling molecules, and variable effects of microglial inhibitor over time, the authors hypothesized that dismutation of superoxide at different time points would reveal different degree of mechanical allodynia in a neuropathic pain model. From the previous reports, using CPIP rat model, the mechanical allodynia was present immediately after reperfusion, peaked at 3 days, and continued for at least 4 weeks after reperfusion with correlated changes of spinal phosphorylated N-methyl-D-aspartate (NMDA) receptor subunit 1 (pNR1) [8,15]. In a preventive paradigm, to study the effect on the development of mechanical allodynia, SOD was treated either before induction of ischemia (preemptive antioxidative treatment) or before reperfusion (dismutation of superoxide at the time of maximal production and interaction). The effect of SOD on existing allodynia, to study the effect on the maintenance of mechanical allodynia, was examined after administration of SOD 3 days after reperfusion (after maximal mechanical allodynia was established). To confirm the superoxide-mediated central sensitization in mechanical allodynia, we evaluated NMDA receptor activation in the spinal cord. Furthermore, to elucidate whether mechanical allodynia is mediated by circulating XO, we performed behavioral testing for each paw.
Adult male Sprague-Dawley rats (280-320 g) were used. Animals were housed in groups of three, with food and water available ad libitum, on a 12:12 h, light:dark cycle. All animals were acclimated in their cages for 7 days before any experiments were performed. All housing conditions and experimental procedures were approved by the Institutional Animal Care and Use Committee, and in accordance with the National Institute of Health guidelines on laboratory animal welfare.
Because sedation or anesthesia might raise mechanical thresholds, it was necessary to determine if these contributed to the behavioral changes interpreted as analgesia. All rats were assessed for posture and righting reflexes, based on the five-point scales described by Kim et al. [2], and all scored 0 at all time points, indicating that the effects due to sedation or anesthesia did not occur.
All chemicals, unless otherwise noted, were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Hindpaw IR injury was induced according to the methods proposed by Coderre et al. [5]. In brief, rats were anesthetized with a bolus (40 mg/kg, i.p.), followed by continuous i.p. infusion of sodium pentobarbital for 2 h (13 mg/h for the first hour, 6.5 mg/h for the second hour). After anesthesia induction, a Nitrile 70 Durometer O-ring (O-rings West, Seattle, WA, USA) with a 7/32-inch internal diameter was placed around the animal's left hind limb, just proximal to the ankle joint. The O-ring remained on the limb for 3 h before it was removed. The termination of sodium pentobarbital anesthesia was coordinated such that the rats recovered fully within 30 min.
Rats were assigned to the three groups (n = 6 per group); Sham, CPIP, or CPIP + Allopurinol. Rats in the CPIP group received 3 h IR injury. For the CPIP + Allopurinol group, rats received 3 h IR injury with i.p. administration of allopurinol (40 mg/kg) 5 min before reperfusion. Rats in the Sham group received neither IR injury nor SOD administration. Thus, the O-ring of the Sham group was cut so that it only loosely surrounded the ankle and did not occlude blood flow to the hindpaw. The lumbar spinal cord (L4-L6) and gastrocnemius muscles of each side (right and left) were used for lipid peroxidation measurement, 3 days following reperfusion.
Malondialdehyde (MDA) levels, an indicator of lipid peroxidation, were measured using the thiobarbituric acid-reactive substances (TBARS) assay [16]. Samples were homogenized in ice-cold sucrose buffer (0.32 M sucrose, 10 mM Tris-HCl, pH 7.4) and then centrifuged. The supernatants were added to a solution containing 0.375% thiobarbituric acid (TBA) and 15% trichloroacetic acid (TCA); the mixture was then heated at 100℃ for 15 min. The MDA concentration for each specimen was determined in a spectrophotometer, based on the absorbance at 532 nm, and was expressed as nM/mg tissue. The concentration of MDA for each animal was recorded as the mean value of the three samples for that animal.
Rats were assigned to five groups (n = 7 per group); Sham, CPIP, Before Ischemia (BI), Before Reperfusion (BR), and After Reperfusion (3AR). SOD (from bovine erythrocyte, 4,000 U/kg, i.p.) was administered 5 min before ischemia (BI), 5 min before reperfusion (BR), or 3 days after reperfusion (3AR). SOD was dissolved in normal saline and prepared immediately before use. Rats in the CPIP group received hindpaw IR injury, without SOD administration (CPIP). Rats in the Sham group received neither IR injury nor SOD administration (Sham), but were injected with an equal volume of vehicle.
Behavioral tests were performed by a tester blind to the experimental conditions. A dynamic plantar aesthesiometer (DPA, Ugo Basile, Comerio, Italy), operated in an automated von Frey method, was used to measure the mechanical allodynia [17]. To aid behavioral observation, rats were placed in a transparent acrylic box with a wire mesh floor. After the animals were completely habituated to the wire mesh (about 15 min), a von Frey filament (straight metal filament, 0.5 mm diameter) was placed on the plantar surface of the paw, and the force (max. 50 g) was increased gradually until a withdrawal response was evoked, and the amount of force needed to cause the withdrawal response was recorded. This procedure was repeated four times with a minimum of 10 s interval and force measurements were averaged. The measurements were performed sequentially in the following order, left hindpaw, right forepaw, right hindpaw, and left forepaw. The baseline testing was performed 1 h before ischemia induction (Bas). After IR injury, behavioral tests were conducted at 24 h (R + 1D) and 3 (R + 3D), 5 (R + 5D), and 7 (R + 7D) days after reperfusion. In the 3AR group, mechanical allodynia was measured 1 h after SOD administration.
The SOD dosage was chosen based on the previous publications. Although there are some drawbacks to the use of SOD in clinical applications, such as rapid clearance and difficulty crossing cell membranes, SOD has demonstrated therapeutic potential for a variety of conditions, including IR injury and inflammation [18]. Local infusion of SOD (4,000 U/kg) reduced both lipid peroxidation and mucosal damage induced by an NO donor [19,20].
In an independent experiment, rats were assigned to five groups (n = 4 per group), similar to those for the behavioral testing. On the third day after reperfusion, the L4-L6 spinal cord was extracted by lumbar laminectomy, separated into the left (ipsilateral) and right (contralateral) cord, and immediately frozen with liquid nitrogen. The spinal cord samples were homogenized in lysis buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 2 mM Na3Vo4, 0.5 mM DTT, 10% Glycerol, 1% Nonidet P-40), containing a protease inhibitor cocktail (Roche, Mannheim, Germany). After centrifuging at 12,000 rpm for 20 min at 4℃, the supernatant protein concentration was quantified using the Bradford method (Bio-Rad, Hercules, USA). Fifty µg of each protein sample was boiled for 5 min at 100℃ in gel a loading buffer (0.5 M Tris-HCl, glycerol, 10% SDS, 0.5% bromophenol blue), separated on 10% SDS-polyacrylamide gels, and then transferred to a nitrocellulose membrane at 60 V for 3 h. The membranes were blocked in 3% non-fat dried milk in Tris buffered saline (50 mM Tris pH 7.4, 10 mM NaCl) for 1 h at room temperature, and then incubated overnight at 4℃ with a primary antibody against phosphorylated NR1, at a dilution of 1 : 500 (Upstate biotechnology, Temecula, USA). After washing with Tris buffered saline (50 mM Tris pH 7.4, 10 mM NaCl), the membranes were incubated for 1 h at room temperature, with peroxidase conjugated secondary antibody (1 : 2,000), and washed again. Proteins were detected using the ECL system (Amersham Biosciences, Buckinghamshire, England). In the 3AR group, the spinal cord was extracted 1 h after SOD administration on the third day after reperfusion.
Data are expressed as the mean ± SEM. Statistical analysis was performed using one-way ANOVA, followed by post-hoc comparisons (Student-Newman-Keuls test). For all statistical analyses, the SPSS software version 13.0 (SPSS, Chicago, IL, USA) was used, and a P value < 0.05 was considered as statistically significant.
In the CPIP group, MDA levels were significantly elevated, 3 days after reperfusion in the spinal cord, and both ipsilateral and contralateral gastrocnemius. In the CPIP + Allopurinol group, allopurinol significantly reduced MDA concentrations in the spinal cord and ipsilateral gastrocnemius, compared to the CPIP group. There were no significant differences in the MDA levels between the Sham and the CPIP + Allopurinol groups (Fig. 1).
Von Frey thresholds for both forepaws did not vary across the 7 days of testing. In addition, there were no differences between the groups (Sham, CPIP, 3AR, BI, and BR), nor within the groups at different time points (Bas, R + 1D, R + 3D, R + 5D, and R + 7D) (Fig. 2A and 2B).
The CPIP group developed mechanical allodynia over a prolonged period in both the ipsilateral and the contralateral hindpaw, with more pronounced effects on the ipsilateral side. Ipsilateral and contralateral mechanical allodynia were present 1 day after reperfusion, peaked at 3 days, and persisted for at least 7 days, following reperfusion. In the 3AR, BI, and BR groups, ipsilateral and contralateral mechanical allodynia were attenuated, compared to the CPIP group 3 days after reperfusion, and this effect persisted for 7 days. Allodynia was most attenuated in BR, followed by BI then 3AR (Fig. 2C and 2D). Data for the Sham group are not shown.
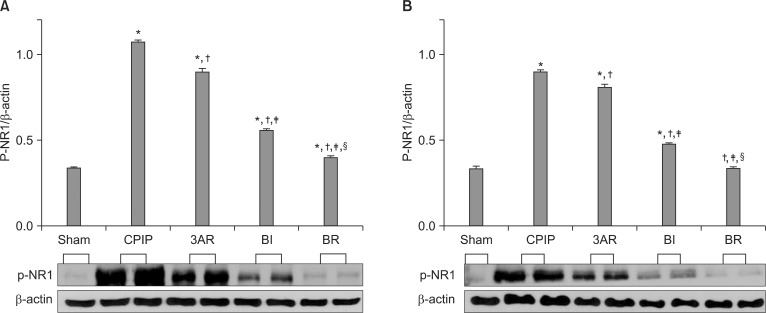
p-NR1 expression (normalized to the β-actin loading control) showed similar changes in the ipsilateral and contralateral spinal cord, with higher p-NR1 expression on the ipsilateral side. NR1 activation was highest in the CPIP, followed by the 3AR, BI, BR, and Sham groups. The degree of NR1 activation in each group was in contrast to the order of withdrawal threshold across groups (Fig. 3).
We examined the effect of superoxide dismutation on the development and maintenance of mechanical allodynia at different time points. Our results reinforced the previous findings that superoxide contributes to the development and maintenance of neuropathic pain. Furthermore, superoxide-mediated mechanical allodynia was closely associated with the central sensitization, measured as increased spinal NMDA receptor activation.
Inhibition of superoxide production before the development phase of neuropathic pain (i.e., the BR and BI groups) attenuated mechanical allodynia and NR1 phosphorylation more effectively than inhibition after allodynia was fully developed (3AR group). The majority of the earlier experiments investigating the mechanical allodynia and ROS were performed in animals with fully developed neuropathic pain [2,21]. Such reports are informative with respect to clinically relevant research. However, those studies focus more on the maintenance of pain rather than on the understanding of the development of the mechanical allodynia. Our data suggest the possible clinical application of preemptive antioxidative treatment in a situation where allodynia is likely to develop, and further the current knowledge of the development of the mechanical allodynia.
The previous literature on IR injury in general has established that superoxide and NO are major contributors to tissue IR injury. Xanthine oxidoreductase (XOR) is a primary source of superoxide [22] and a key source of ROS in tissue IR injury [23]. Both animal models and clinical studies have shown that, XO, an oxidase form of XOR, can serve as a key source of ROS, which contributes to the inflammatory signaling, IR injury, and impaired vascular function. During ischemia, XOR is converted predominantly to XO and high concentrations of hypoxanthine accumulate as AMP is catabolized. The reintroduction of molecular oxygen into ischemic tissue, upon reperfusion, leads to the XO-mediated overproduction of ROS, particularly superoxide. It is well established that superoxide can initiate the lipid peroxidation [24]. In contrast, allopurinol inhibits (hypo)xanthine oxidation at the molybdenum core of XO, decreasing superoxide production. In this study, we indirectly confirmed superoxide production using an allopurinol-induced inhibition of lipid peroxidation in the CPIP model.
Clarification of the time course of superoxide contribution to the mechanical allodynia is crucial for understanding the mechanisms of allodynia development, as well as for developing optimal treatment strategies. In the present study, we administered SOD at three time points: before ischemia, before reperfusion, and 3 days after reperfusion. In the BI group, SOD was administered before an IR injury and superoxide production. Thus, this group received preemptive antioxidative treatment as in preemptive analgesia. As maximum superoxide production occurs during reperfusion, the BR group is most representative of the effects of superoxide on mechanical allodynia. The 3AR group reflects the effects of SOD administration when neuropathic-like pain is already developed. These data have implications for the clinically relevant effects of antioxidants, as well as the effects of superoxide on the maintenance phase of mechanical allodynia. In the development phase of CPIP, superoxide inhibition attenuated mechanical allodynia and p-NR1 activation, an effect that persisted into the maintenance phase. These findings suggest that superoxide is closely associated with the development and maintenance of the mechanical allodynia via central sensitization. Kwak et al. [8] reported lasting effects of antioxidants injected during the early phase of neuropathic-like pain in the CPIP model. In that study, allopurinol administered during the initial period of an IR injury showed a clear and persistent anti-allodynic effect. Notably, increased superoxide production decreases NO bioavailability. Thus, the anti-nociceptive effect of SOD in this study might be due not only to the effects on the superoxide, but also to a decreased NO bioavailability. NO can exert pro- or anti-nociceptive effects, depending on the concentration and the stage of pathological processes [25].
Our data suggest that mechanical allodynia is mediated through central sensitization, not via systemic circulating XO. Sensitization of dorsal horn cells in the spinal cord (central sensitization) plays a fundamental role in neuropathic pain. We evaluated the effects of systemic circulating XO on the development of the mechanical allodynia, through the behavioral testing on the forepaws and hindpaws. In clinical IR injury, a variety of factors may result in elevated circulating XO levels, which can then lead to XO deposition in remote vascular beds, following a high-affinity endothelial glycosaminoglycan binding and subsequent endocytosis of the catalytically active enzyme [10]. Thus, the XO produced during ischemia enters the systemic circulation after reperfusion, and is then transported to distant regions of the body. XO released from a reperfused limb may produce high levels of superoxide in the immediate vicinity of the limb. However, some XO may reach more remote areas, including the forepaws and contralateral hindpaw, resulting in superoxide production in those regions. Accordingly, mechanical allodynia would develop not only in the affected paw, but also in other paws. In this study, the contralateral hindpaw showed a similar pattern of allodynia, as the ipsilateral hindpaw. Furthermore, changes in NR1 phosphorylation in the contralateral hindpaw were similar to those of the ipsilateral hindpaw, and were correlated with behavioral changes. However, neither of the forepaws showed any changes upon behavioral testing. These results indicate that central sensitization is the primary contributor to the mechanical allodynia in the CPIP model.
With respect to the maintenance of mechanical allodynia, an SOD mimetic (M40403) not only prevented the development of inflammation and hyperalgesia, after injection of an inflammatory agent into the rat paw, but also reduced existing hyperalgesia [26]. In fully developed neuropathic pain produced by the rat spinal nerve ligation model, the effect of the free radical scavenger phenyl-N-tert-butylnitrone (PBN; 50 mg/kg, three injections at 4 h intervals) was graded with repeated injections; mechanical allodynia was attenuated 1 h after PBN injection [2]. Moreover, systemic injection of PBN (100 mg/kg, i.p.) reduced hyperalgesia and blocked the increases in spinal p-NR1 in both neuropathic and inflammatory pain models 1 h after PBN treatment [21]. In the present study, SOD administered to the 3AR group (where maximal mechanical allodynia was produced) 1 h before the behavioral test significantly decreased the mechanical allodynia and p-NR1 activation, although to a lesser degree than in the BI or BR groups. Hence, our results reinforce previous findings that suggest that ROS, and superoxide in particular, participate in the maintenance of the nociceptive cascade and the nociceptor sensitization process.
Reperfusion of a limb, following 3 h of ischemia, will result in the release of XO and subsequent XO-mediated superoxide production. According to the previous reports, increased XO levels persist for less than 1 h [27]. Thus, in the 3AR group, SOD dismutased superoxide that was produced by a non-XO-mediated mechanism. Although superoxide is a common target of SOD in both development and maintenance phases of allodynia, it is likely that the sources of superoxide during the two phases are different. During the development phase, superoxide is produced by XO; during the maintenance phase, it may result from NMDA receptor activation. Supporting this idea, superoxide is produced upon NMDA receptor stimulation in cultured cerebellar granule cells [28], and an NMDA receptor antagonist, MK-801, blocks superoxide generation [29]. Moreover, in a rat model of spinal cord injury, decreased SOD and increased MDA were reversed by a NMDA receptor blocker [30]. These data suggest that NMDA receptor activation induces superoxide production, which may in turn, accelerate the central sensitization during the maintenance of the mechanical allodynia. Accordingly, we propose that the anti-allodynic effect of SOD in the 3AR group was mediated by a reduced NMDA receptor activation due to superoxide inhibition by the administered SOD.
In conclusion, superoxide dismutase significantly reduced the mechanical allodynia and reversed NMDA receptor activation in the CPIP model. Preemptive antioxidative treatment was also effective in reducing the development and maintenance of mechanical allodynia, although it was less effective than administered immediately prior to superoxide production. Antioxidative treatment during the maintenance phase was effective, but likely involves different mechanisms than in the development phase. In addition, allodynia was mediated by central sensitization, not by circulating XO-mediated superoxide. These data suggest that superoxide is important for the development and maintenance of chronic post-ischemia pain, particularly with respect to the central sensitization in the spinal cord. Thus, both preemptive superoxide inhibition and superoxide inhibition after the mechanical allodynia is developed may represent effective therapeutic avenues for the treatment of persistent pain.
References
1. De Alba J, Clayton NM, Collins SD, Colthup P, Chessell I, Knowles RG. GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain. 2006; 120:170–181. PMID: 16360270.

2. Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004; 111:116–124. PMID: 15327815.

3. Eisenberg E, Shtahl S, Geller R, Reznick AZ, Sharf O, Ravbinovich M, et al. Serum and salivary oxidative analysis in Complex Regional Pain Syndrome. Pain. 2008; 138:226–232. PMID: 18539395.

4. Perez RS, Zuurmond WW, Bezemer PD, Kuik DJ, van Loenen AC, de Lange JJ, et al. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003; 102:297–307. PMID: 12670672.

5. Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004; 112:94–105. PMID: 15494189.

6. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999; 80:539–544. PMID: 10342415.

7. Laferrière A, Millecamps M, Xanthos DN, Xiao WH, Siau C, Mos MD, et al. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Mol Pain [serial on the internet]. 2008. 10. 2008 Oct 28. Available from http://www.molecularpain.com/content/4/1/49.

8. Kwak KH, Han CG, Lee SH, Jeon Y, Park SS, Kim SO, et al. Reactive oxygen species in rats with chronic post-ischemia pain. Acta Anaesthesiol Scand. 2009; 53:648–656. PMID: 19419360.

9. Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006; 391:108–111. PMID: 16183198.

10. Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, et al. Binding of xanthine oxidase to vascular endothelium. kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999; 274:4985–4994. PMID: 9988743.
11. Ji R, Xu Z, Wang X, Lo EH. MMP-2 and MMP-9-investigations in neuropathic pain phases. US Neurol. 2008; 4:71–74.

12. Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003; 23:4017–4022. PMID: 12764087.

13. Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005; 114:149–159. PMID: 15733640.

14. Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003; 306:624–630. PMID: 12734393.

15. Kim KW, Ha MJ, Jung KY, Kwak KH, Park SS, Lim DG. Reactive oxygen species and N-methyl-D-aspartate receptor-mediated central sensitization in hindlimb ischemia/reperfusion injury-induced neuropathic pain rats. Korean J Anesthesiol. 2009; 56:186–194.

16. Lee JH, Park JW. A manganese porphyrin complex is a novel radiation protector. Free Radic Biol Med. 2004; 37:272–283. PMID: 15203198.

17. Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008; 28:2892–2902. PMID: 18337420.

18. Francis JW, Ren J, Warren L, Brown RH Jr, Finklestein SP. Postischemic infusion of Cu/Zn superoxide dismutase or SOD:Tet451 reduces cerebral infarction following focal ischemia/reperfusion in rats. Exp Neurol. 1997; 146:435–443. PMID: 9270054.

19. Lamarque D, Whittle BJ. Role of oxygen-derived metabolites in the rat gastric mucosal injury induced by nitric oxide donors. Eur J Pharmacol. 1995; 277:187–194. PMID: 7493608.

20. Lamarque D, Whittle BJ. Involvement of peroxynitrite in the lipid peroxidation induced by nitric oxide in rat gastric mucosa. Eur J Pharmacol. 1996; 313:R5–R7. PMID: 8905346.

21. Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007; 131:262–271. PMID: 17317010.

22. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969; 244:6049–6055. PMID: 5389100.
23. Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981; 81:22–29. PMID: 6263743.

24. McCord JM. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Response. 2008; 6:223–238. PMID: 18846257.

25. Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997; 20:132–139. PMID: 9061868.

26. Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004; 309:869–878. PMID: 14988418.

27. Trewick AL, el-Hassan K, Round JM, Adiseshiah M. Xanthine oxidase in critically ischaemic and claudicant limbs: profile of activity during early reperfusion. Br J Surg. 1996; 83:798–802. PMID: 8696745.

28. Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993; 364:535–537. PMID: 7687749.

29. Li L, Shou Y, Borowitz JL, Isom GE. Reactive oxygen species mediate pyridostigmine-induced neuronal apoptosis: involvement of muscarinic and NMDA receptors. Toxicol Appl Pharmacol. 2001; 177:17–25. PMID: 11708896.

30. Vural M, Arslantas A, Yazihan N, Koken T, Uzuner K, Arslantas D, et al. NMDA receptor blockage with 2-amino-5-phosphonovaleric acid improves oxidative stress after spinal cord trauma in rats. Spinal Cord. 2010; 48:285–289. PMID: 19668258.

Fig. 1
Malondialdehyde (MDA) concentrations in the spinal cord (L4-L6) and gastrocnemius muscles in the Sham, CPIP, and CPIP + Allopurinol groups (n = 6 per group). CPIP rats received 3 h of IR injury. In the CPIP + Allopurinol group, rats received 3 h of IR injury and allopurinol (40 mg/kg, i.p.) 5 min before reperfusion. Rats in the Sham group received neither IR injury nor SOD administration. The MDA concentration for each animal was recorded as the mean value of the three samples from that animal. In the CPIP group, MDA was significantly increased in the spinal cord and both the ipsilateral and contralateral gastrocnemius 3 days after reperfusion. Allopurinol reversed the increase in MDA, indicating allopurinol-sensitive, xanthine oxidase-mediated superoxide production. The data are expressed as the mean ± SEM. *Indicates values significantly different from Sham. †Indicates values significantly different from CPIP. P < 0.05 was considered statistically significant. CPIP: chronic post-ischemia pain.
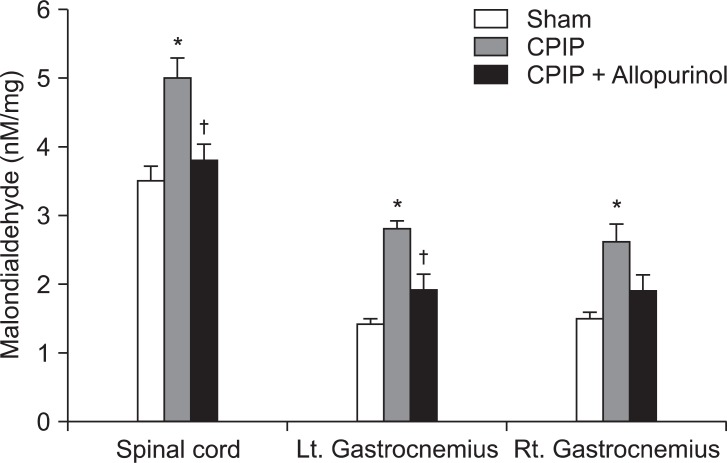
Fig. 2
Changes in the withdrawal thresholds for the left forepaw (A), right forepaw (B), left hindpaw (C), and right hindpaw (D). Rats were assigned to five groups (n = 7 per group); the Sham, CPIP, after reperfusion (3AR), before ischemia (BI), and before reperfusion (BR) groups. Superoxide dismutase (4,000 U/kg, i.p.) was administered 5 min before ischemia (BI), 5 min before reperfusion (BR), or 3 days after reperfusion (3AR). Rats in the CPIP received IR injury but not SOD treatment. Rats in the Sham group received neither IR injury nor SOD administration (data not shown). The withdrawal thresholds were measured in the following order: left hindpaw, right forepaw, right hindpaw, and left forepaw. Rats were tested for baseline thresholds 1 h before ischemia induction (Bas). Behavioral testing was performed 24 h (R + 1D), 3 (R + 3D), 5 (R + 5D), and 7 (R + 7D) days after reperfusion. In the 3AR group, mechanical allodynia was measured 1 h after SOD administration. Von Frey thresholds for both forepaws did not vary across the 7 days of testing. Ipsilateral and contralateral mechanical allodynia were attenuated the most in the BR group, followed by the BI and 3AR groups. Data are expressed as mean ± SEM. *Indicates values significantly different from CPIP. †Indicates values significantly different from 3AR. ‡Indicates values significantly different from BI. §Indicates values significantly different from Bas in the same group. P < 0.05 was considered statistically significant. CPIP: chronic post-ischemia pain.
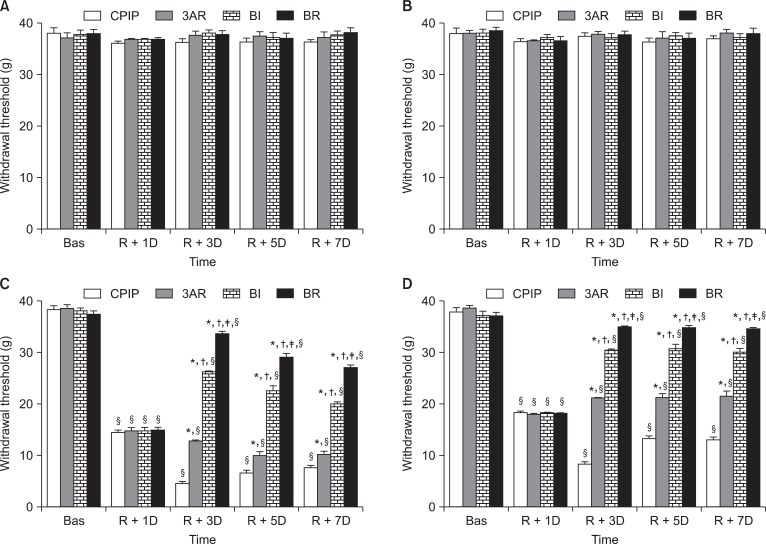
Fig. 3
A representative Western blot and averaged phosphorylation values of the NMDA receptor subunit1 (p-NR1) in the Sham, CPIP, 3AR, BI, and BR groups (n = 4 per group) 3 days after reperfusion. Superoxide dismutase (4,000 U/kg, i.p.) was administered 5 min before ischemia (BI), 5 min before reperfusion (BR), or 3 days after reperfusion (3AR). Rats in the CPIP group received IR injury, but not SOD treatment. Rats in the Sham group received neither IR injury nor SOD administration. On the third day after reperfusion, the L4-L6 spinal cord was extracted by lumbar laminectomy, separated into left (A) and right (B) cord, and analyzed by Western blotting. In the 3AR group, the spinal cord was extracted 1 h after SOD administration on the third day after reperfusion. The relative density of p-NR1 was the highest in the CPIP group and decreased in the order of the 3AR, BI, BR, and Sham groups. Each value shown on the graph represents the average (± SEM) gel density ratio for each group. β-actin was used as an internal control. *Indicates values significantly different from Sham. †Indicates values significantly different from CPIP. ‡Indicates values significantly different from 3AR. §Indicates values significantly different from BI. P < 0.05 was considered statistically significant. CPIP: chronic post-ischemia pain.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download