Introduction
Although cancer treatment with chemotherapeutic agents, such as taxanes, vinca alkaloids, and platinum complexes increases patient survival times, side effects may diminish quality of life. Paclitaxel (PAC) is a widely used chemotherapeutic drug for treating breast cancer, cervical cancer, ovarian cancer, non-small cell lung carcinomas, and Kaposi's sarcoma. PAC binds to β-tubulin of microtubules, stabilizing microtubules and enhancing microtubule polymerization [
1]. PAC induces a dose-limiting side effect, neuropathic pain, which is characterized by numbness, tingling, and burning. The mechanisms producing this neuropathic pain are not fully understood [
2,
3].
Many factors influence the incidence of chemotherapy-induced peripheral neuropathy, including patient age, dose intensity, cumulative dose, therapy duration, co-administration of other chemotherapeutic agents, and pre-existing conditions. Fifty-five percent of patients receiving a cumulative dose of 200 mg/m
2 PAC developed sensory disturbances compared to only 30% receiving 175 mg/m
2 [
3].
Investigating chemotherapy-induced neuropathic pain is particularly difficult, because neuropathic pain may arise from multiple causes (chemotherapy, tumor effects, radiotherapy, surgical trauma, etc.). Animal models present a simpler situation. PAC-induced neuropathic pain (PINP) in rats is a commonly used model of chemotherapy-induced neuropathic pain. PAC-treated rats have pronounced mechanical allodynia, cold allodynia, and mechanical hyperalgesia but little or no heat hyperalgesia [
2]. Nerve trauma models produce pronounced heat hyperalgesia. The observation that drug-induced neuropathic pain is related to an abnormality in mitochondria within sensory axons has been reported [
4]. Flatters and Bennett [
5] suggested that PAC induces degeneration or dysfunction of axonal microtubules without axonal degeneration or disruption of the axonal cytoskeleton. Massive axonal degeneration occurs in the nerve trauma model [
6]
PAC is frequently used in female patients, but most animal experiments of PINP have been conducted with male rats or mice. Gender is a critical factor modulating the experience of pain. Males and females experience pain differently and respond differentially to specific classes of analgesic medications. A quantitative review of the literature regarding gender differences of experimental pain responses concluded that females show greater sensitivity to several modalities of experimental pain than males [
7]. However, the relationship between pain behavior and gender differences in PINP has not been studied, and the gender differences in the clinical incidence of neuropathic pain after PAC administration have not been reported.
The objective of this study was to investigate the gender difference in pain behavior after a PAC injection in rats and to compare the effect of analgesics in this pain model.
Materials and Methods
Materials
Sprague-Dawley rats (n = 24), weighing 150 to 250 g at the start of the experiments, were used. Two rats per cage were housed under standard laboratory conditions with controlled room temperature (23℃) and a 12-hour light/dark cycle. This experimental protocol was approved by the Institutional Animal Care and Use Committee.
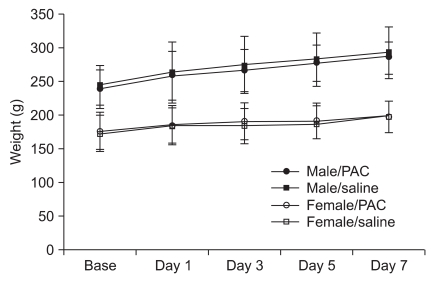
Animals were divided into two groups of males (n = 12) and females (n = 12). Each group was subdivided into a saline group (n = 4 each) and a PAC group (n = 8 each). Body weight was recorded on each experimental day.
Injection of PAC and development of neuropathic pain
Rats were placed in a Plexiglas box and anesthetized with 4% isoflurane and oxygen. After induction of anesthesia, rats were randomly injected intraperitoneally with 1 ml/kg saline or 1 ml/kg PAC (Taxol; Bristol-Myers-Squibb, New York, NY, USA, 2 mg/ml). PAC was diluted with saline to create a 2 mg/ml concentration that was injected intraperitoneally (2 mg/kg) on 4 alternate days (days 1, 3, 5, and 7). Body weight was recorded before the PAC or saline injections.
Behavioral testing
The behavioral test was conducted by an examiner who was blinded to the groups. The measured test was the foot withdrawal threshold in response to mechanical stimuli applied to the hind paws. Rats were placed in a Plexiglas chamber with a mesh floor. Before testing, rats were habituated to the testing environment for more than 15 minutes. Baseline measurements were taken prior to PAC administration. The plantar surface of the hind paw (sciatic nerve territory) was tested with a calibrated von Frey filament (3.65, 3.87, 4.10, 4.31, 4.52, 4.74, 4.92, and 5.16). The von Frey filament was applied perpendicularly to the most sensitive areas of the plantar surface at the center area of the paw or the base of the third or fourth toes with sufficient force to bend the filament slightly for 2-3 seconds. Abrupt withdrawal of the foot during stimulation or immediately after stimulus removal was considered a positive response.
The up-down method of Dixon [
8] was used for testing the withdrawal threshold. The first stimulus was always the 4.31 filament. When there was a positive response, the next lower filament was used, and when no response was observed, the next higher filament was applied. This testing pattern continued until responses to the sixth von Frey stimuli from the first change in response were measured. The responses were then converted into a 50% threshold value using the formula: 50% threshold = 10
(X + kd)/10
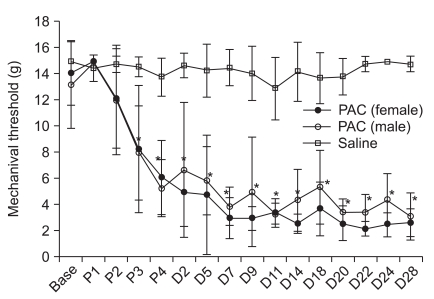
4, where X is the value of the final von Frey hair in log units, k is the tabular value for the pattern of positive or negative responses, and d is the mean difference between stimuli in log units (0.22). When negative or positive responses were still observed for the 3.84 or 5.18 filament, values of 0.3 or 15.0 g were assigned, respectively, by assuming a value of ± 0.5 for k. Testing was conducted on the day PAC was injected, and 2, 5, 7, 9, 11, 14, 18, 20, 22, 24, and 28 days after the fourth PAC injection.
Responses to analgesic drugs
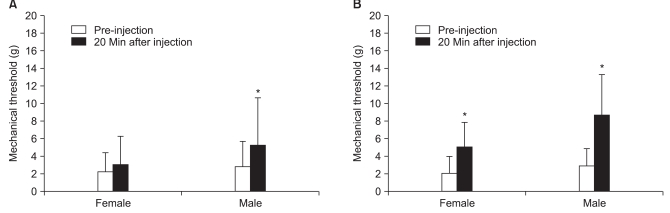
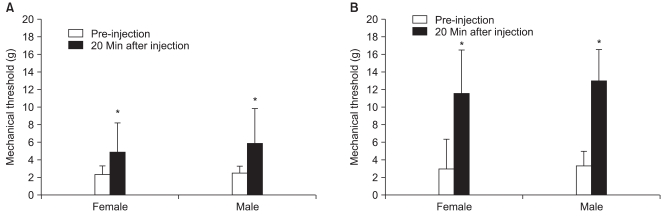
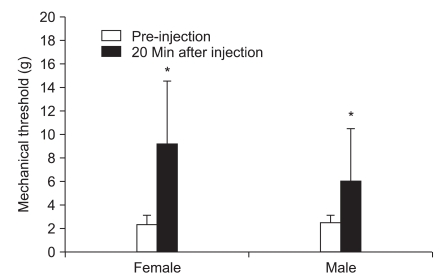
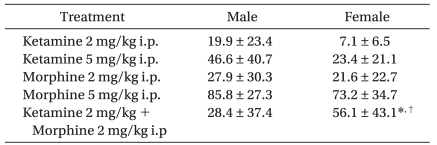
The responses to analgesics were determined after intraperitoneal (i.p.) administration of 2 mg/kg ketamine, 5 mg/kg ketamine, 2 mg/kg morphine, 5 mg/kg morphine, and 2 mg/kg morphine plus 2 mg/kg ketamine at 32-60 days after PAC injection. Drugs were diluted with saline, and the concentration was adjusted to make an equal volume of 1 ml/kg. Mechanical thresholds of the right hind paws were measured before and 20 minutes after intraperitoneal injection of each drug. A 7-day treatment interval was used to avoid overlap. The pre-injection withdrawal threshold was checked to confirm the absence of the previous treatment.
Statistical analysis
Data are presented as mean ± standard deviation. The Statview 5.0 program (SAS Institute Inc., Cary, NC, USA) was used to test statistical significance. The withdrawal thresholds of each group were compared with a repeated-measures analysis of variance followed by Scheffe's post hoc test. The demographic data and responses to the analgesic drugs were compared with the Mann-Whitney U and Wilcoxon signed-rank tests. A P < 0.05 was considered statistically significant.
Discussion
In this PAC-induced neuropathic pain model, the mechanical threshold decreased on the day of the third PAC injection and was maintained until the end of the experiment. No gender difference was observed for the withdrawal threshold in PAC-treated rats The PINP mechanism has not been revealed, and there are no validated drugs for prevention and treatment. Several experimental animal models of chemotherapy-related neurotoxicity have been developed to reveal the mechanism. The present study was conducted using the PAC model of Polomano et al. [
2]. Some authors have suggested that chemotherapy-induced painful neuropathy is accompanied by ultrastructural changes in unmyelinated sensory neurons, including cytoskeletal disorganization and microtubule disorientation. One study indicated that the pain is associated with dysfunction in Aβ, Aδ, and C caliber primary afferent fibers [
9]. Massive axonal degeneration occurs in post-traumatic painful peripheral neuropathy models, whereas animals with PINP have no axonal degeneration at the level of the peripheral nerve [
10,
11]. PAC-treated rats have pronounced mechanical allodynia, cold allodynia, and mechanical hyperalgesia, but little or no heat hyperalgesia [
2]. The nerve trauma models, such as the chronic constrictive injury model, produce the same symptoms but also produce pronounced heat hyperalgesia. Different mechanisms suggest the possibility of different pain responses and pharmacological response profiles.
Women are more likely than men to report acute and chronic pain [
12] and use pain relieving medication significantly more often, even when pain frequency and severity is equaled between the genders [
13]. Biological, cultural, psychological, and social factors are all hypothesized to contribute to these disparities in pain responses, reporting, and management [
7]. In animals, most studies of gender differences in pain have examined acute nociception. In the partial nerve injury model, female rats are more susceptible to develop spread mechanical hypersensitivity, particularly after a facial nerve injury, compared with male rats. However, hypersensitivity of the hind paws after a sciatic nerve injury does not significantly differ between female and male rats [
14], suggesting that gender differences in the neuropathic pain in rats depend on the site of injury and the type of test.
In this study, an intraperitoneal injection of 2 mg/kg or 5 mg/kg of morphine increased the mechanical threshold in both genders dose dependently. Opioid analgesic drugs are widely used for chronic pain, and morphine produces greater analgesia in males compared to that in female rats [
15]. Three opioid receptors, µ, κ, and δ, are significantly implicated in modulating pain [
16]. The µ receptor is the most clinically relevant receptor, as morphine and most other opioid agonists act preferentially at that site; thus, studies have focused most attention on this opioid receptor. In animal studies, the intensity of noxious stimulation used during pain testing influences the observed gender effect. Female predominance of µ-opioid analgesia is most notable at low to moderate intensities in thermal studies [
17]. However, no significant difference in the anti-allodynic effect of morphine between male and female rats was observed in this study.
Cata et al. [
18] reported that PINP in rats is accompanied by changes in sensory processing by spinal cord neurons as evidenced by increased spontaneous activity, which is prolonged after discharges due to natural mechanical and thermal stimuli and increased windup to cutaneous electrical stimuli. Similar changes are observed in rats with vincristine-induced painful neuropathy [
10], chronic constriction neuropathy, and partial nerve injury neuropathy [
19], as well as in several models of central sensitization. Increased spontaneous activity in dorsal horn neurons has been hypothesized to result from increased discharge in peripheral neuromas or pathophysiological dorsal root ganglia neurons due to denervation supersensitivity, disinhibition, synaptic reorganization, post-synaptic activation of
N-methyl-D-aspartate (NMDA) receptors, and cyclooxygenase type-2 induction [
20]. An NMDA antagonist would be helpful to diminish after-discharge in the wide dynamic range neurons. In general, low dose ketamine does not produce an analgesic effect [
21]. A maximally tolerated dose of MK-801 results in incomplete reversal of mechanical allodynia in PINP [
22]. However, low dose ketamine (2 mg/kg) showed an analgesic effect in male rats in the present study. In females, 5 mg/kg ketamine resulted in a notable increase in the withdrawal threshold compared to that of 2 mg/kg ketamine, suggesting that NMDA activation may be related to gender and the mechanism of neuropathic pain.
It has been established that the glutamate system interacts with the opioid system. Opioids affect NMDA receptors, and NMDA receptors are involved in the development of opioid tolerance [
23]. NMDA antagonists may potentiate opioid analgesia while preventing or slowing the development of opioid tolerance and dependence [
24]. However, reports of NMDA antagonist modulation on the acute analgesic effects of opioid agonists vary from enhancement, to no effect, to attenuation [
25-
27]. In this study, 2 mg/kg ketamine enhanced the antinociceptive effect of 2 mg/kg morphine only in females. Some controversies exist as to the gender differences in the modulation of NMDA antagonists. Dextromethorphan enhances morphine analgesia in males but attenuates it in female rats [
28]. In an acute pain model, there is enhancement of morphine antinociception by NMDA antagonist in both genders, but no effect is observed in females in a chronic pain model [
29]. However, ketamine (3 mg/kg) produces a threefold increase in morphine (3 mg/kg) analgesia in female rats, whereas increase in morphine analgesia is negligible in male rats [
21], which corresponds with the present study. These gender differences in the NMDA-opioid interaction are influenced by differences in neuroendocrine physiology, neurotransmitter density, and the physiological response to steroids [
15,
21]. The hippocampus of male rats contains significantly more AMPA, kainate, and NMDA receptors than those of female rats. Female rats in diestrus have significantly higher AMPA receptor densities than female rats in estrus [
30]. These conflicting data show that morphine potentiation by NMDA antagonists follows a complex mechanism that may depend on the antinociceptive assay used, the pain model, and other factors such as type of antagonist, dose, and gender.
The effect of chemotherapy on body weight varies based on the type of agent and administered dose. A volume of data indicates that chemotherapy-treated rats fail to gain weight during the treatment period [
10,
11]. However, no significant change was observed between the normal saline group and the PAC-injected group in the current study. Rats treated with PAC (2 mg/kg, days 1, 3, 5, 7) gained weight normally. The PAC dose was selected in a range that would induce neuropathic pain without other complications.
The present study did not include any information about the effect of the estrous cycle or gonadal hormones. Therefore, more studies are required to understand the exact mechanism and other factors. Further research to obtain more information about gender differences of other therapeutic drugs may aid in the development of better treatment for PINP.
In summary, no difference in the incidence or severity of PINP was observed between male and female rats. No significant difference was observed in the analgesic effects of morphine or ketamine on the PINP. Low dose ketamine (2 mg/kg i.p.) alone was not effective for increasing the withdrawal threshold in females. However, a notable enhancement of the analgesic effect was shown when administered in combination with morphine in females. The results suggest that ketamine may play a significant role in the treatment of PINP when combined with opioids, particularly in females.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download