Abstract
Antiphospholipid syndrome (APS) is defined as an autoimmune disorder characterized by recurrent thrombosis or obstetrical morbidity. A 29-year-old woman who was diagnosed with APS underwent emergency cesarean delivery at 23 weeks' gestation. She had a seizure attack and her laboratory findings were: AST/ALT 1459/1108 IU/L, LDH 1424 IU/L, 30% hematocrit, a platelet count of 43 × 103/ml and urine protein (4+). We describe the anesthetic experience of catastrophic HELLP syndrome with antiphospholipid syndrome and we review the relevant literature.
Among the 10-12% of pregnancies complicated with pre-eclampsia or eclampsia, the incidence of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome has been estimated at 0.01-0.2% in the general population [1]. Antiphospholipid syndrome (APS) is defined as an autoimmune disorder characterized by recurrent thrombosis or obstetrical morbidity including recurrent abortion, pre-eclampsia, eclampsia and placental insufficiency. These clinical features are associated with the presence of auto-antibodies against negatively charged phospholipids or phospholipid-binding proteins [2]. The risk of HELLP syndrome might be increased in APS but, its incidence is difficult to estimate. Additionally, HELLP syndrome appears more severe in APS than in the general population [1]. In previous reports, the severe form of HELLP syndrome with hepatic infarction and bone marrow necrosis were associated with APS [3,4].
Anesthesiologists are involved with the care of these pregnancies with a high risk of thrombosis, bleeding tendencies, cerebral infarction and psychiatric problems. [5]. We report an anesthetic experience with catastrophic HELLP syndrome and antiphospholipid syndrome.
A 29-year-old woman (height, 164 cm; weight, 85 kg; gravid 2; parity 0) underwent cesarean delivery at 23 weeks' gestation. She had a history of antiphospholipid syndrome diagnosed by her rheumatologist 11 years ago. She experienced deep vein thrombosis with antiphospholipid antibodies and was lupus anticoagulant positive in that time. She was taking Astrix (100 mg/day). Six days before operation, she was admitted due to persistant epigastric pain. Blood pressure was 120/80 mm Hg, heart rate was 72 beats/min and body temperature was 36.8℃. Blood laboratory data were as follows: serum aspartate aminotransaminase (AST) 549 IU/L, serum alanine aminotransaminase (ALT) 489 IU/L, serum lactate dehydrogenase (LDH) 790 IU/L, blood urea nitrogen (BUN) 17.5 mg/dl, creatinine 0.5 mg/dl, hematocrit 29.7%, platelet count 54 × 103/ml, prothrombin time (PT) 12 sec and activated partial thromboplastin time (aPTT) 82.4 sec. A urine specimen demonstrated proteinuria (1+). Transabdominal ultrasound showed weak portal vein flow (main, 18.4 cm/sec; right, 30 cm/sec; left, 29 cm/sec) with a small amount of ascites. The obstetrician suggested autoimmune hepatitis or portal vein thrombosis was complicating antiphospholipid syndrome rather than HELLP syndrome because of the early gestational period and normal blood pressure. Repeated daily blood chemistries revealed an aPTT of 41-60 sec and therefore 1,000 unit/hour of heparin was initiated to reach the target aPTT of 80-100 seconds and 40 mg of prednisolone was added as treatment. For evaluating autoimmune hepatitis, auto-antibodies were checked. Anti-cardiolipin antibodies were examined by enzyme linked immunosorbent assay (ELISA). The IgG (51.8 GPL units/ml) was positive and IgM (2.76 MPL units/ml) was negative. A lupus anticoagulant test was also positive (control ratio of 2.67). Other laboratory investigations were unremarkable. On the morning of the operation, she had abrupt vomiting with generalized tonic-clonic seizures with eyeball deviations of 30-40 sec; she recovered spontaneously. One hour after the 1st seizure attack, she had another seizure attack with high blood pressure (200/100 mmHg); hydralazine and diazepam was given. The laboratory findings revealed AST/ALT 1,459/1,108 IU/L, LDH 1,424 IU/L, hematocrit 30%, platelet count 43 × 103/ml, aPTT 87.6 sec and urine protein (4+).
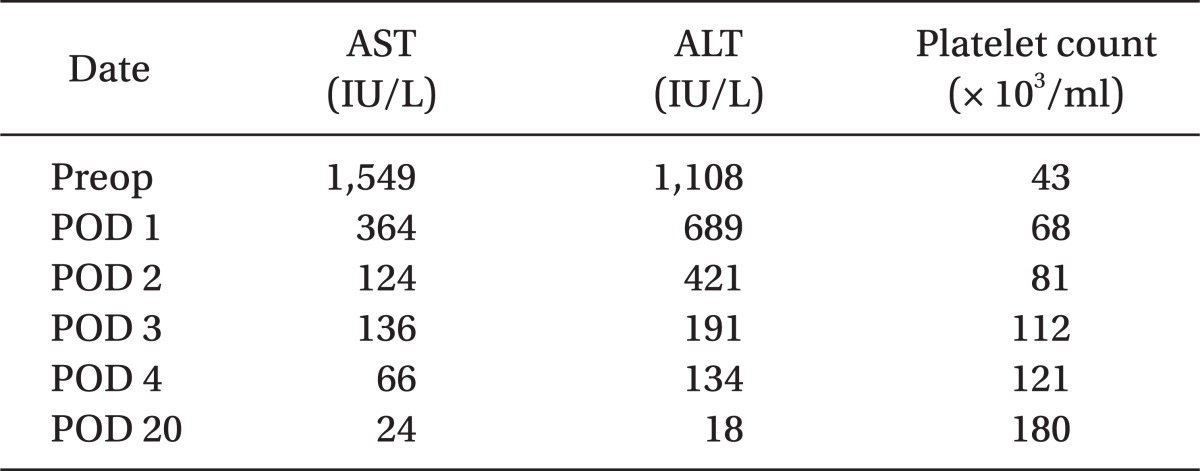
An emergency cesarean delivery was scheduled, she was not premedicated and upon arrival to the operating room, standard monitoring devices were applied. Vital signs were blood pressure 190/120 mmHg, heart rate 110 beats/min and pulse oxymetry revealed 99% oxygen saturation before anesthetic induction. The patient already received heparin and therefore, the patient did not wear a compression stocking as this could increase arterial blood pressure with increased venous return. Anesthesia was induced with 300 mg of pentothal sodium and 50 mg of rocuronium. Tracheal intubation was performed using a single lumen internal diameter (ID) 6.5 mm tube in one attempt without difficulty. Anesthesia was maintained with sevoflurane (1-2%) within BIS between 30-60. Systolic blood pressure was maintained about 140-160 mmHg using intravenous nicardipine (100-300 ug bolus) and body temperature was maintained about 36.8-37.3℃. A cesarean delivery was performed by a Pfannenstiel incision and a 470 gram live-born male infant was delivered (APGAR score; at 1 min, 3 and at 5 min, 7). Intravenous magnesium sulfate (170 mg/hour) and alfentanyl (1,000 ug) were given to the patient. The cesarean delivery proceeded without an event. The patient received fentanyl (1,000 ug) in 100 ml of normal saline for 48 h using patient controlled intravenous analgesia. At the end of surgery, all anesthetic agents were discontinued and the residual neuromuscular blockade was reversed with pyridostigmine and glycopyrrolate. Estimated blood loss was 500 ml and she was not transfused with blood components. The tracheal tube was removed when the patient responded to verbal commands and showed sufficient spontaneous respiration and neuromuscular function. There were no signs of uterine atony or bleeding. Pathologic observation of the placenta revealed mulifocal infarction involving up to 40% of the placenta. The patient remained in the post-anesthetic care unit for 1 hour event free and was then transferred to the intensive care unit. Intravenous magnesium sulfate used for 24 hours and she was transferred to the general ward at postoperative day 1. She had an uneventful recovery with improved liver function and platelet counts (Table 1) and was discharged on postoperative day 8.
APS was first described in 1983 and requires the following clinical findings to diagnose: venous or arterial thrombosis, recurrent pregnancy loss and/or thrombocytopenia, together with moderate to high levels of IgG and/or IgM anticardiolipid antibodies or a positive lupus anticoagulant test [6]. Although obstetric problems include pre-eclampsia, eclampsia and placental thrombosis with premature delivery frequently occurring in these patients, HELLP syndromes complicating APS reports are rare and this paper is unique in a Korean woman.
HELLP syndrome is a thrombotic microangiopathic state with thrombocytopenia and occurs usually before 32 weeks of gestation. There is some overlap for treatment between APS and HELLP syndrome. First is the beneficial effects of the administration of corticosteroids. Steroid can minimize the degree of intravascular endothelial injury and improve blood flow while diminishing hepatocyte death and platelet consumption in HELLP syndrome [3]. In any autoimmune disorder, corticosteroids also improve clinical signs and it is already known that the beneficial effects of corticosteroids in catastrophic APS [7].
A second similarity is the use of heparin. Antiphospholipid antibodies react with serum protein beta 2-glycoprotein and the phospolipid complex which normally is responsible for inhibiting factor XII activation, platelet activation and prothrombinase activity. Interfering with these mechanisms could predispose to thrombosis in APS, despite the of prolongation of aPTT [8]. In HELLP syndrome, an endothelial/trophoblastic dysfunction and a low-grade activation of coagulation in the microcirculation are likely to play a major role in the intravascular coagulation in the utero-placental vascular bed and liver. Heparin might prevent the continuing formation of microthrombi and controlling the cause of the intravascular hemolysis and the thrombocytopenia [9].
While low-dose heparin does not increase the risk of hemorrhage, this is premised on the assumption of normal aPTTs and platelet function [10]. In the retrospective study of 119 patients with HELLP syndrome, 87% of patients were anesthetized with a neuroaxial block (82% of epidural anesthesia, 5% of spinal anesthesia). Among them 12 patients who received a neuroaxial block had platelet counts less than 50 × 103/ml without neurologic and hematolic complications or bleeding in the epidural space. Thus, Vigil-De Gracia et al. [11] recommended that neuroaxial anesthesia may be safely administered in the patients with HELLP syndrome without disseminated intravascular coagulopathy or prolonged PT/aPTT. However, our patient had both low platelet counts and prolonged aPTT while on heparin. We cannot find any report about the safety of this situation, so we performed general anesthesia rather than regional anesthesia.
We used both corticosteroid and heparin to reach the target aPTT of between 80 and 100 seconds. However, antiphospholipid antibodies could prolong the aPTT or activated clotting time. The thromboelastogram or measuring the level of blood heparin concentrations is recommended for evaluating the function of this anticoagulant [12]. We could not use other parameters since our case was an emergency situation.
In a previous report, ante-partum HELLP syndrome occurred in only 18% of the cases before 27 weeks of gestation [13]. However, in a literature review of the 24 cases of HELLP syndrome complicating APS, HELLP syndrome occurred before 27 weeks' gestation in 67% of the patients [1]. In this case, if we judged HELLP syndrome on eclampsia earlier, we could start proper management such as intravenous magnesium or anti-hypertensive therapy before the seizure attack. Because of early gestation and atypical signs, a clinician misjudged the case as autoimmune hepatitis or portal vein thrombosis complicating antiphospholipid syndrome rather than HELLP syndrome.
In our case, the obstetrician used hydralazine for the 2nd seizure attack with hypertension. This drug can cause drug-induced lupus and thrombotic complications associated with lupus anticoagulation with prolonged treatment at high doses [14]. Although there are no reports of drug-induced lupus and thrombotic complication with single bolus use, it might not have been a good choice for this case because of slow onset of the acute hypertensive event and the patient's underlying disease. Nicardipine can be a good choice because the actions of nicardipine on calcium metabolism result in an antithrombotic effect through an increased availability of vasodilating eicosanoids in vessel walls and through a reduced amount of prothrombotic substances [15].
Although most HELLP syndromes resolve with delivery and supportive care, it sometimes leads to maternal death [13]. Our case was HELLP syndrome with eclampsia complicating APS. In the APS patients, HELLP syndrome can be more severe and sometimes refractory. Therefore, even in the earlier signs and symptoms of HELLP syndrome, we have to think about the possibility of complicating APS, and have to prevent the subsequent complication by using heparin and steroids.
References
1. Le Thi Thuong D, Tieulie N, Costedoat N, Andreu MR, Wechsler B, Vauthier-Brouzes D, et al. The HELLP syndrome in the antiphospholipid syndrome: retrospective study of 16 cases in 15 women. Ann Rheum Dis. 2005; 64:273–278. PMID: 15647435.

2. Heilmann L, Schorsch M, Hahn T, Fareed J. Antiphospholipid syndrome and pre-eclampsia. Semin Thromb Hemost. 2011; 37:141–145. PMID: 21370215.

3. Asherson RA, Galarza-Maldonado C, Sanin-Blair J. The HELLP syndrome, antiphospholipid antibodies, and syndromes. Clin Rheumatol. 2008; 27:1–4. PMID: 17912576.

4. Sinha J, Chowdhry I, Sedan S, Barland P. Bone marrow necrosis and refractory HELLP syndrome in a patient with catastrophic antiphospholipid antibody syndrome. J Rheumatol. 2002; 29:195–197. PMID: 11824961.
5. Madan R, Khoursheed M, Kukla R, al-Mazidi M, Behbehani A. The anaesthetist and the antiphospholipid syndrome. Anaesthesia. 1997; 52:72–76. PMID: 9014551.

6. Ringrose DK. Anaesthesia and the antiphospholipid syndrome: a review of 20 obstetric patients. Int J Obstet Anesth. 1997; 6:107–111. PMID: 15321291.

7. Gordon A, McLean CA, Ryan P, Roberts SK. Steroid-responsive catastrophic antiphospholipid syndrome. J Gastroenterol Hepatol. 2004; 19:479–480. PMID: 15012797.

8. McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Antiphospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein 1 (apolipoprotein H). Proc Natl Acad Sci USA. 1990; 87:4120–4124. PMID: 2349221.
9. Brain MC, Kuah KB, Dixon HG. Heparin treatment of hemolysis and thrombocytopenia in pre-eclampsia. Report of a case and a review of the literature. J Obstet Gynaecol Br Commonw. 1967; 74:702–711. PMID: 6058533.
10. Howell PR, Douglas MJ. Lupus anticoagulant, paramyotonia congenital and pregnancy. Can J Anaesth. 1992; 39:992–996. PMID: 1451229.
11. Vigil-De Gracia P, Silva S, Montufar C, Carrol I, De Los Rios S. Anesthesia in pregnant women with HELLP syndrome. Int J Gynaecol Obstet. 2001; 74:23–27. PMID: 11430937.

12. Hogan WJ, McBane RD, Santrach PJ, Plumhoff EA, Oliver WC Jr, Schaff HV, et al. Antiphospholipid syndrome and perioperative hemostatic management of cardiac valvular surgery. Mayo Clin Proc. 2000; 75:971–976. PMID: 10994834.

13. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am J Obstet Gynecol. 1993; 169:1000–1006. PMID: 8238109.

14. Cameron HA, Ramsay LE. The lupus syndrome induced by hydralazine: a common complication with low dose treatment. Br Med J (Clin Res Ed). 1984; 289:410–412.

15. Cignarella A, Bertozzi D, Zaarour C, Puglisi L. Antithrombotic activity of nicardipine in spontaneously hypertensive rats. Pharmacol Res. 1994; 30:273–280. PMID: 7862621.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download