Abstract
A 48-year-old woman with cystic fibrosis and a previous left pneumonectomy had surgery planned for single lung transplantation under general anesthesia. Due to progressive dyspnea and recurrent respiratory infection, she could not maintain her normal daily life without lung transplantation. The anesthetic management and surgical procedure was expected to be difficult because of the left mediastinal shift and an asymmetric thorax after the left pneumonectomy, but the single lung transplantation was successfully done under cardiopulmonary bypass.
Since the first human received a lung transplant in 1963 [1], refined surgical techniques, the development of immunosuppressive drugs, and the expert care provided after surgery have increased the survival rate for lung transplant patients. According to the data from the International Society for Heart and Lung Transplantation in 2005, the one-year survival rate was 75% and 5-year survival rate was 50% [2]; thus, lung transplantation is considered an effective treatment for end stage lung disease.
For patients with cystic fibrosis, bilateral sequential lung transplantation is safe and is regarded as the best option because bilateral sequential lung transplantation is a favorable way for an early and long-term prognosis [3]. However, single lung transplantation for patients who had a pneumonectomy is an unusual case with the first report in 1997 [4,5] and in that case, it was different from existing single lung transplantation in that there were a mediastinal shift and an asymmetric thorax, and it required extracorporeal circulation after the pneumonectomy. In regards to this, the authors experienced the anesthetic management of a patient with cystic fibrosis who had previously undergone a pneumonectomy on the opposite side. The authors report about this case along with a literature review.
A 48-year-old woman 155 cm tall weighing 30 kg, with cystic fibrosis had surgery planned for single lung transplantation. Twenty-years earlier, she was diagnosed with cystic fibrosis. She needed right lung transplantation because of progressive dyspnea and recurrent respiratory infection after left pneumonectomy, which was due to a huge aspergilloma in the upper lobe of the left lung 5 years before her visit to our hospital. There was nothing particular about her case except for her lung disease and her vital signs and laboratory workup were normal before surgery. In addition, arterial blood gas analysis (pH 7.35, PaCO2 51.8 mmHg, PaO2 74.6 mmHg, HCO3- 28 mEq/L, BE 1.3 mEq/L, SaO2 94.2%) showed mild respiratory acidosis. Results of the echocardiography before surgery showed a left ventricular ejection fraction of 69%, an enlargement of the right atrium and ventricle, a mild tricuspid regurgitation, severe pulmonary hypertension, and minimal pericardial effusion. The donor was a patient with traumatic SAH and SDH and was fit for donating a lung based of their medical history and laboratory workup.
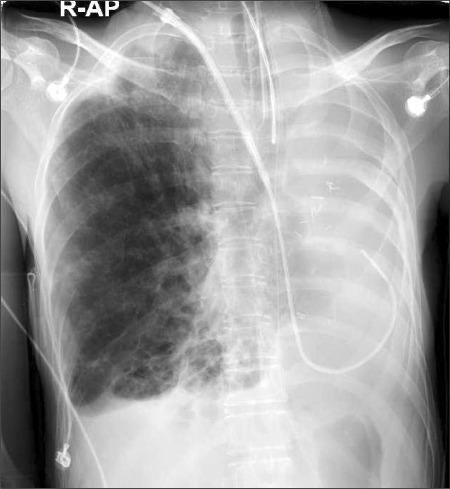
The cystic fibrosis patient had an enlargement of the right thorax and a left mediastinal shift (organs such as trachea, heart, aorta, vena cava etc.) as a result of the past left pneumonectomy, and it caused an abnormal positioning, twisting, and narrowing of the subclavian vein and internal jugular vein; therefore, a 9 Fr. central vein catheter (advanced venous access, AVA high flow device, Edwards LifeSciences, USA) was inserted into the internal jugular vein under angiography in the angiography room because of difficulty in performing the catheter insertion and due to the risk of vascular perforation (Fig. 1).
The patient arrived at the operating room and was administered oxygen 5 L/min by nasal cannula. Additionally, noninvasive BP monitoring, electrocardiography, and pulse oxymetry was immediately done after arriving at the operating room, and a BIS monitor was attached and a monitor was connected to the right radial artery catheter and a femoral artery catheter. Before putting the patient under anesthesia, her measured vital signs were a BP of 115/78 mmHg, a HR of 85 beats/min, and a SaO2 of 95%. To induce anesthesia, 6 mg of etomidate and 5 mg of vecuronium were injected and an internal diameter 7.0mm tracheal tube was inserted after manual ventilation with 100% oxygen. The ventilator was set to a FiO2 of 0.5, a PEEP of 5 cmH2O, a RR of 10 breaths/min and a TV of 350 ml using the volume-controlled mode. After that, through the center vein catheter, which was inserted into the right internal jugular vein, a 7 Fr. pulmonary artery catheter (Swan-Ganz CCOmbo catheter, Edwards LifeSciences, USA) was inserted into the central pulmonary artery, and at that time, the pulmonary artery pressure was 61/26 mmHg. To maintain anesthesia, 3.5 µg/ml of propofol and 3.0 ng/ml of remifentanil with target-controlled infusion were consistently injected and the injection speed of these anesthetics was controlled according to the change in vital signs, and cell saver was used during the surgery.
After positioning the patient in the supine position with both arms elevated, the surgery started with a clamshell incision. First of all, before the pneumonectomy, by inserting a catheter into the aorta and the right atrium, cardiopulmonary bypass was done during surgery to not only prevent unstable vital signs due to severe pulmonary hypertension and profuse bleeding caused by severe adhesion of the right lung as a result of recurring infection but also to oxygenize the blood after the pneumonectomy. Before the cardiopulmonary bypass was started, 10,000 units of heparin were injected, and 3 minutes later, the activated coagulation time was 450-seconds. After starting the cardiopulmonary bypass, the ventilator was taken off and 1,000 mg of methylprednisolone were injected after the pneumonectomy. Thereafter, immediately, the bronchus, pulmonary artery, and vein of the transplanted lung were anastomosed to the patient. At some point, blood pressure dropped 80/45 mmHg because of bleeding or pressing of the intramediastinal structure, but it was relatively stable maintaining a blood pressure of more than 100/50 mmHg and SaO2 of 100%. The ischemic time of the right lung was 75-minutes.
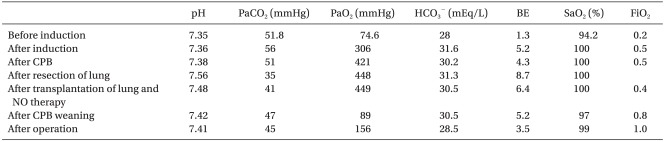
Anastomosis of each organ was done well and reperfusion was attempted after pulmonary vein anastomosis. To reduce increased pulmonary artery pressure and prevent reperfusion injury, a weaning off of the cardiopulmonary bypass was attempted after setting the FiO2 to 0.4, the PEEP to 5 cmH2O, the RR to 14 breaths/min, the TV to 300 ml, and the nitric oxide (NO) to 20 ppm by the volume-controlled mode connected to a prepared NO ventilator. The results of the arterial blood gas analyses after connecting the NO ventilator were stable showing a pH of 7.48, a PaCO2 of 41 mmHg, a PaO2 of 449 mmHg, a HCO3- of 30.5 mEq/L, a BE of 6.4 mEq/L, and a SaO2 of 100%. The results of the arterial blood gas analyses after weaning off of the cardiopulmonary bypass was a pH of 7.42, a PaCO2 of 47 mmHg, a PaO2 of 89 mmHg, a HCO3- of 30.5 mEq/L, a BE of 5.2 mEq/L, and a SaO2 of 97%; therefore, a FiO2 of 1.0 was maintained. Table 1 presents the results of the arterial blood gas analyses during the surgery.
The blood pressure decreased to 75/40 mmHg during the process of reducing the flow to gradually wean the patient off the cardiopulmonary bypass; thus, fluids were injected into her body, and 10 mcg/kg/min and 0.02 mcg/kg/min of dopamine and epinephrine, respectively, were consistently injected. After that, the surgery was completed with no complications and the patient was transferred to the SICU with manual ventilation. Total anesthesia time was 6 hours 45 minutes; the operation lasted 4 hours; cardiopulmonary bypass time was 2 hours 25 minutes. The volume of total bleeding was 3,000 ml; the amount of total fluid infused was 500 ml of crystralloid solution and 1,100 ml of colloid solution. Total transfusion was 10 units of irradiated WBC-depleted RBC, 7 units of FFP, 10 units of PC. Urine output was 1,600 ml.
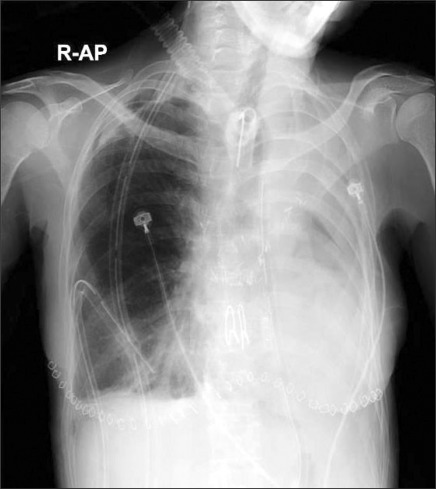
2 days after the surgery, the patient received a tracheostomy to take her off the ventilator (Fig. 2). Six days after surgery, she was transported to the general ward and seemed to get better using a home ventilator. However, 42 days later, she died of inhalation pneumonia.
Patients who have end stage lung diseases such as cystic fibrosis, bronchiectasia along with secondary lung infections need lung transplantation to prolong their life. In general, there are different kinds of lung transplantations such as heart-lung transplantation, bilateral (en bloc, sequential) lung transplantation, and single lung transplantation. Usually, bilateral lung transplantation or heart-lung transplantation is required [6,7] because a newly transplanted lung can be infected from the remaining infected lung. The patient in this case who had undergone left pneumonectomy before had a right single lung transplantation. Single lung transplantation has become an acceptable treatment modality for patients with a previous pneumonectomy. It helps to extend patients' lives and improve their quality of life even though there are high early morbidities and mortalities [4].
The main purpose of anesthesia for lung transplantation is early extubation because of the side effects of mechanical ventilation associated with lung colonization, pressure injury, leakage at the bronchial anastomosis site, and narcotics requirement for sedation. To achieve this, it is required to use BIS during anesthesia to inject medicines at the necessary minimum density to avoid excessive anesthesia related to delayed emergence or light anesthesia linked with awareness during surgery [8].
There are some critical points to be aware of for anesthesia of lung transplant patients. It is necessary to pay attention to the medications given to lung transplant patients because there are risks of low blood pressure due to a chronic reduction of vessel capacity. In addition, in some cases, inducing anesthesia should be performed in a sitting posture because of patients who cannot lie flat on their back. Moreover, light anesthesia should be avoided because it can cause a severe increase in the pulmonary artery pressure and bronchospasm. Besides, excessive administration of fluids during surgery should positively be avoided because it frequently causes pulmonary edema in the newly transplanted lung [9,10].
In addition, for patients who had a pneumonectomy, techniques for and patient care after the surgery can be difficult due to several complications like empyema, bronchopleural fistula, pulmonary edema after pneumonectomy, postpneumonectomy syndrome which is characterized by proximal airway obstruction because of stretching or compressing of the distal trachea, and main or lobar bronchi stretching and compression against the descending aorta or thoracic spine [11], but these complications were not observed in this case.
Two points that should be considered different from the anesthesia of single lung transplantation for patients who had an opposite pneumonectomy to the anesthesia of general bilateral lung transplantation or single lung transplantation are first the asymmetric thorax and mediastinal shift. It is possible to have some trouble in inserting the center vein catheter for anesthesia management and surgical procedures. Correct positioning of the aortic arch and right atrial cavity and the distortion of the vena cava can affect the incision method for the operation and the cannulation site for extracorporeal circulation. Therefore, a change in the vessel structures caused by an asymmetric thorax and mediastinal shift should be analyzed entirely before the surgery and a computed tomography (CT) scan to check for abnormalities in the structures is the best method to establish a plan for pneumonectomy and anticipate difficulties before and after surgery [4]. In this case, the chest CT before surgery showed that the patient had a left mediastinal shift and an asymmetric thorax so it was anticipated that it would be difficult to use a blind central venous catheterization method. Therefore, the patient was rolled into the operating room after central venous catheterization was done under angiography in the angiography room.
The second point is ventilation is impossible from after pneumonectomy to until anastomosis completion because of the absence of an opposite lung. This time, extracorporeal circulation was required to maintain proper oxygen saturation and cardiopulmonary bypass was done because of the patient's pulmonary artery hypertension, the difficulty of the lung dissection caused by the mediastinal shift, the adhesion of the pleura and lung, the expectation of excessive bleeding, and the severe change in blood pressure due to continuous manipulation of the mediastinal structures. Cardiopulmonary bypass becomes an indication when there is pulmonary artery hypertension and right heart failure or difficulty in maintaining gas exchange during one lung ventilation in surgery [9].
In this situation, transesophageal echocardiography (TEE) is helpful to determine the necessity of extracorporeal circulation and also it makes it possible to recognize important incidents like functional abnormalities of the right ventricle and tricuspid valve, ventricular septal deviation, intracardiac air embolism, stenosis and thrombus of the anastomosis site of the pulmonary vessel and atrium, and to do fast therapeutic interventions during lung transplantation [12]. The strong point of cardiopulmonary bypass is to improve the site of dissection for the operation by reducing heart pressure and collapsing the lung, and to have a low possibility of hemodynamic and respiratory instability, and to reduce a transplanted lung's incidence rate of infection for patients who had infectious lung diseases by providing a clean surgical environment [13].
However, cardiopulmonary bypass has a high possibility of increasing the risk of pulmonary edema and lung injury caused by the administration of excessive fluid and blood transfusion due to the possibility of bleeding and by increased inflammatory response due to the extracorporeal circulation; therefore, it cause to increase the duration of mechanical ventilation after the operation, and cardiopulmonary bypass is associated with early graft dysfunction and increased mortality [14]. Because of these negative points, cardiopulmonary bypass should be avoided if it is at all possible. However, if it is inevitable to do cardiopulmonary bypass, then the administration of corticosteroids for suppressing the release and expression of cytokines and a heparin coated circuit for attenuating the formation of complement complex substances should be used as precautions [15]. For the patient in this case, preventive corticosteroids were used to reduce the side effects of the cardiopulmonary bypass.
In conclusion, when single lung transplantation for patients who previously had a pneumonectomy are performed, general anesthesia for lung transplantation can be applied; however, a thorough check of the patient and preparation of the anesthesia by recognizing the anatomical changes caused by an asymmetric thorax and a mediastinal shift before surgery should be done. In addition, all surgical equipment for the surgery should be handled proficiently. Based on that, patients should be in surgery with the greatest of care.
References
1. Hardy JD. The first lung transplant in man (1963) and the first heart transplant in man (1964). Transplant Proc. 1999; 31:25–29. PMID: 10083000.

2. Taylor DO, Edwards LB, Boucek MM, Trulock EP, Deng MC, Keck BM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty second official adult heart transplant report-2005. J Heart Lung Transplant. 2005; 24:945–955. PMID: 16102427.
3. Vergnat M, Farhat F, Tronc F, Jegaden O. Metachronous single lung transplanation after contralateral pneumonectomy. A "big" challenge? Minerva Chir. 2007; 62:187–190. PMID: 17519844.
4. Le Pimpec-Barthes F, Thomas PA, Bonnette P, Mussot S, DeFrancquen P, Hernigou A, et al. Single-lung transplantation in patients with previous contralateral pneumonectomy: technical aspects and results. Eur J Cardiothorac Surg. 2009; 36:927–932. PMID: 19632853.

5. Piotrowski JA, Splittgerber FH, Donovan TJ, Ratjen F, Zerkowski HR. Single-lung transplantation in a patient with cystic fibrosis and an asymmetric thorax. Ann Thorac Surg. 1997; 64:1456–1458. PMID: 9386721.

6. Shennib H, Massard G, Gauthier R, Colman N, Mulder D. Cystic Fibrosis Transplant Study Group. Single lung transplantation for cystic fibrosis: is it an option? J Heart Lung Transplant. 1993; 12:288–293. PMID: 8476903.
7. Forty J, Hasan A, Gould FK, Corris PA, Dark JH. Single lung transplantation with simultaneous contralateral pneumonectomy for cystic fibrosis. J Heart Lung Transplant. 1994; 13:727–730. PMID: 7947892.
8. Liu N, Chazot T, Trillat B, Michel-Cherqui M, Marandon JY, Law-Koune JD, et al. Closed-loop control of consciousness during lung transplantation: an observational study. J Cardiothorac Vasc Anesth. 2008; 22:611–615. PMID: 18662642.

9. Haddy S, Bremmer R. Update on anesthesia for lung transplantation. Sem Anesth Periop Med Pain. 2004; 23:34–41.

10. Rosenberg AL, Rao M, Benedict PE. Anesthetic implications for lung transplantation. Anesthesiol Clin North America. 2004; 22:767–788. PMID: 15541935.

11. Grillo HC, Shepard JA, Mathisen DJ, Kanarek DJ. Postpneumonectomy syndrome: diagnosis, management and results. Ann Thorac Surg. 1992; 54:638–650. PMID: 1417220.

12. Serra E, Feltracco P, Barbieri S, Forti A, Ori C. Transesophageal echocardiography during lung transplantation. Transplant Proc. 2007; 39:1981–1982. PMID: 17692671.

13. Pochettino A, Augoustides JG, Kowalchuk DA, Watcha SM, Cowie D, Jobes DR. Cardiopulmonary bypass for lung transplantation in cystic fibrosis: pilot evaluation of perioperative outcome. J Cardiothorac Vasc Anesth. 2007; 21:208–211. PMID: 17418733.

14. Dalibon N, Geffroy A, Moutafis M, Vinatier I, Bonnette P, Stern M, et al. Use of cardiopulmonary bypass for lung transplantation: A 10-year experience. J Cardiothorac Vasc Anesth. 2006; 20:668–672. PMID: 17023286.

15. McRae K. Con: lung transplantation should not be routinely performed with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000; 14:746–750. PMID: 11139122.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download