Abstract
Background
Benzodiazepines have a hypnotic/sedative effect through the inhibitory action of γ-aminobutyric acid type A receptor. Flumazenil antagonizes these effects via competitive inhibition, so it has been used to reverse the effect of benzodiazepines. Recently, flumazenil has been reported to expedite recovery from propofol/remifentanil and sevoflurane/remifentanil anesthesia without benzodiazepines. Endogenous benzodiazepine ligands (endozepines) were isolated in several tissues of individuals who had not received benzodiazepines.
Methods
Forty-five healthy unpremedicated patients were randomly allocated to either flumazenil or a control groups. Each patient received either a single dose of 0.3 mg of flumazenil (n = 24) or placebo (n = 21). After drug administration, various recovery parameters and bispectral index (BIS) values in the flumazenil and control groups were compared.
Results
Mean time to spontaneous respiration, eye opening on verbal command, hand squeezing on verbal command, extubation and time to date of birth recollection were significantly shorter in the flumazenil group than in the control group (P = 0.004, 0.007, 0.005, 0.042, and 0.016, respectively). The BIS value was significantly higher in flumazenil group than in the control group beginning 6 min after flumazenil administration.
Conclusions
Administration of a single dose of 0.3 mg of flumazenil to healthy, unpremedicated patients at the end of sevoflurane/fentanyl anesthesia without benzodiazepines resulted in earlier emergence from anesthesia and an increase in the BIS value. This may indicate that flumazenil could have an antagonistic effect on sevoflurane or an analeptic effect through endozepine-dependent mechanisms.
Benzodiazepines can cause sedation and amnesia by affecting the central nervous system. The effect of benzodiazepines is exerted at the specific binding site of the γ-aminobutyric acid type A (GABA-A) receptor. The receptor is responsible for a major inhibitory effect on anesthesia [1,2]. Flumazenil, a benzodiazepine antagonist, has a dose-dependent antagonistic effect on all of the actions of benzodiazepines, including sedation, amnesia and respiratory depression via competitive inhibition at the benzodiazepine binding site on the GABA-A receptor [3]. For this reason, flumazenil is used for the purpose of antagonism in general anesthesia when a benzodiazepine is used [4], but recently, a few studies have been performed testing the effect of flumazenil on recovery from general anesthesia in which no benzodiazepines were used. For example, there have been reports demonstrating a positive effect of flumazenil injection on recovery from general anesthesia that did not include benzodiazepine medication [5,6]. Additionally, a few studies have reported on the effect of inhalation anesthetics, including sevoflurane, on the GABA-A receptor [7-9]. Therefore, we conducted this study with the assumption that flumazenil may affect the recovery parameters and BIS of sevoflurane/fentanyl general anesthesia.
This study included 45 patients at or below the age of 67 and with an American Society of Anesthesia physical status class 1 or 2, who were undergoing an operation expected to take one or two hours. Those whose body mass index was lower than 18 kg/m2 or higher than 30 kg/m2, those who had diseases that might affect the level of consciousness, such as stroke and dementia, those who had been undergoing treatment for a cardiovascular disease or taking a sedative or somnifacient, and those whose blood loss was expected to be more than 1 L were excluded from this study. This study was approved by the Hospital Ethics Committee. An explanation of the study was provided to all subjects and written consents were received from them before the operation. Except for sex, there were no significant differences in the demographic data between the two groups including body mass index, preanesthetic systolic and diastolic blood pressure, heart rate, oxygen saturation, duration of operation, blood loss and fentanyl dose (Table 1).
The subjects were not premedicated. After arrival in the operating room, the subjects' blood pressure, heart rate, electrocardiograph, peripheral oxygen saturation and BIS were continuously measured using a patient monitoring instrument. Before the induction of anesthesia, glycopyrrolate 0.2 mg was injected intravenously. Thiopental sodium (4 mg/kg), fentanyl (2 µg/kg), rocuronium (0.6 mg/kg) and sevoflurane (2-4 vol/%) were used for anesthetic induction. An esophageal thermometer was inserted to monitor body temperature, and train-of-four stimulation (TOF) monitoring was performed. Oxygen and nitrous oxide were administered each at the rate of 1.5 L/min, and the expiratory sevoflurane concentration was kept at 1.5 vol/%. The anesthesia was maintained while keeping the expiratory carbon dioxide pressure at 30-40 mmHg. The BIS was maintained at around 40 throughout the operation. In order to maintain the hemodynamic parameters, including blood pressure and heart rate, within 20% of those measured before the induction, fentanyl (0.5-1.0 µg/kg) was injected if necessary during the operation. To maintain T1 in the TOF ratio (T4 : T1) at 25%, rocuronium (0.2 mg/kg) was intravenously injected with the top-up dose. The patient's fluid levels were maintained with crystalloid solution. The blood loss, calculated by measuring the weight of aspiratory vessels and gauze, was compensated with 6% hydroxyethyl starch (130/0.4) (Voluven, Fresenius Kabi, Bad Homburg, Germany). A heating blanket (Bair Hugger Warming unit-Model. 505, Arizant healthcare, USA) was used to keep the patient's body temperature at or higher than 36℃.
End of the operation was the last skin suture and an intravenous injection of fentanyl (0.5 µg/kg) was given five minutes before the expected end of the operation while sevoflurane was continuously administered without reducing the concentration for pain alleviation. The reversal of muscular relaxation (TOF 0.9) was induced using pyridostigmine. At the end of the operation, sevoflurane and nitrous oxide were stopped and flumazenil (0.3 mg, in 3 ml volume) or normal saline (3 ml) was intravenously injected while oxygen supply was increased and hyperventilation was performed. Doctors and nurses of the Anesthesiology and Pain Medicine Department participating in the anesthesia were blinded to treatment.
After termination of the anesthesia, the time taken to spontaneous respiration while attempting to awaken the patient with the same words in 20-second intervals as well as hand squeezing and eye opening on verbal command, extubation of the endotracheal tube and recollection of their date of birth were measured; BIS values were also recorded in two-minute intervals. The patient was then transferred to the recovery room and carefully monitored for one hour by medical staff who had not participated in the anesthesia. The patient was discharged from the recovery room and transferred to the ward according to general standards.
Statistical analysis was performed using SPSS software (version 18.0, SPSS Inc., Chicago, IL). The parameters related to the recovery from general anesthesia and the BIS values were expressed as means ± standard deviation (SD). A normality test was performed with respect to each of the parameters to compare the time to reach the index representing recovery from anesthesia between the two groups. After verifying that the parameters satisfied normality, a two-sample t-test was performed. To compare the BIS values over time between the two groups, we performed a two-way ANOVA followed by a Bonferroni posthoc test. A P value of less than 0.05 was considered significant.
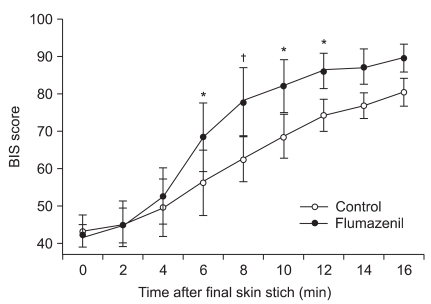
The mean time taken to spontaneous respiration, hand squeezing and eye opening on verbal command, extubation of the endotracheal tube and recollection of their date of birth was significantly shorter in the flumazenil injection group than in the control group (Table 2). The BIS values at zero, two, and four minutes after the intravenous injection of flumazenil or normal saline at the end of the operation were not significantly different, but the values at six, eight, ten, and twelve minutes were significantly higher in the experimental group than in the control group. The values did not show a significant difference at 14 and 16 minutes after the injection (Fig. 1).
Flumazenil, which has an antagonist effect on benzodiazepines, can be used when a patient is overly sedated from excessive medication with benzodiazepines or fails to recover from amnesia following an operation [8]. Specifically, this effect of flumazenil is caused by competitive inhibition of the GABA-A receptor, a target for benzodiazepines [4]. In this study, sevoflurane/fentanyl anesthesia was carried out in unpremedicated patients who were not given benzodiazepines and the results showed that injection of flumazenil at the end of anesthesia significantly reduced the time taken to recovery from the anesthesia and increased the BIS value when compared to the control group.
A previous study had shown that flumazenil itself does not have such an effect: 0.3 mg of flumazenil was injected to a control group or a group that had received midazolam and the auditory and somatosensory evoked cortical reaction was increased only in the midazolam-treated group, with no changes in the non-injection group [10]. Thus, it can be presumed that flumazenil may have the effect to help recovery from anesthesia by antagonizing certain mechanism(s) of general anesthesia.
Several studies have reported on the antagonistic effect of flumazenil on anesthetics other than benzodiazepines. Weinbroum and Geller [11] injected flumazenil for recovery from halothane, enflurane and isoflurane anesthesia, and reported an improvement in the cognitive and motor abilities as well as the subjective feelings of the patients. Roald et al. [12] found a significant increase in the cerebral metabolic rate of oxygen (CMRO2) when flumazenil was injected to dogs anesthetized with isoflurane, but not in the non-anesthetized dogs and concluded that flumazenil has a partial antagonizing effect on isoflurane. Recently, Dahaba et al. [5] reported that flumazenil injection enhanced the recovery from propofol/remifentanil anesthesia and significantly increased the BIS value.
On the other hand, there are studies reporting the opposite result. In an experiment with mice, Murayama et al. [13] reported that flumazenil did not antagonize the effects of halothane, thiamylal and propofol. Schwieger et al. [14] injected flumazenil at doses ranging from 0.5-4.5 mg/kg in dogs anesthetized with enflurane, isoflurane and enflurane/fentanyl, and reported that the injection did not affect the minimal alveolar concentration (MAC) of the inhalation anesthetics or the plasma fentanyl concentration. Moreover, Schwartz et al. [15] reported that flumazenil injection in dogs anesthetized with isoflurane anesthesia may play the role of an agonist and not an antagonistin reducing the MAC.
Another hypothesis is that flumazenil might help recovery from anesthesia by antagonizing intrinsic benzodiazepines (or benzodiazepine-like ligands), not extrinsic benzodiazepines. The spontaneous endozepines or intrinsic benzodiazepine receptor ligands were extracted from various mammalian tissues that had not been exposed to benzodiazepines [16]. In particular, intrinsic benzodiazepines were detected in the cerebrospinal fluid, plasma and serum of humans who had not taken benzodiazepines, and even in the breast milk of a healthy woman who had never taken benzodiazepines immediately after child-birth [17-20]. Additionally, there was a report that a considerable amount of benzodiazepine-like ligands were detected in the brain of a hepatic encephalopathy patient who had not previously been exposed to benzodiazepines [20]. A later study conducted on the basis of this result showed that injection of flumazenil to a hepatic encephalopathy patient who had not been given benzodiazepines resulted in electroencephalographic improvements [21].
According to a previous study on the effect of flumazenil injection on general anesthesia performed without using benzodiazepines, injection of 0.5 mg of flumazenil 30 minutes before the end of the operation, in a patient undergoing general anesthesia with propofol/remifentanil, resulted in the increase of the BIS value and later had a positive effect on the parameters for recovery from anesthesia [5]. In our study, 0.3 mg of flumazenil was injected just before the end of the operation and there was a significant [positive] effect on the recovery, but the effect was smaller than in the study mentioned previously. Although it is not accurately known how propofol and sevoflurane act on the GABA-A receptor to produce the anesthetic effect, a recent study suggested that they have different active sites on the GABA-A receptor [22]. When flumazenil is clinically used to antagonize benzodiazepines, it takes effect in about 1-5 minutes. If flumazenil is injected at 1 mg to obtain the desired consciousness level, the effect is maintained for about 2 hours and the duration is proportional to the dose [23]. If the faster recovery from anesthesia [in the studies we discuss here] was achieved by flumazenil antagonizing the actions of propofol or sevoflurane on the GABA-A receptor, the difference in the results can be explained by the fact that the two anesthetics have different active sites and the dose was different. Moreover, the significant increase of the BIS value in the experimental group in comparison to the control group at six minutes after flumazenil injection in the present study might have been because of the rapid expression of the flumazenil effect. The difference in the BIS value was no longer significant from 14 minutes after the injection, which might have been because the effect of the small amount of flumazenil used (0.3 mg) disappeared and the sevoflurane used for the anesthesia was no longer present in both groups.
Different from the study where the injection was performed 30 minutes before the end of the anesthesia, there was a study in which flumazenil was injected just before the end of the operation to investigate the effect on recovery, but only the time taken to the appearance of the recovery parameters was measured, without measuring the BIS value [6]. Since BIS decreases in a dose-dependent manner with respect to various anesthetics such as propofol, sevoflurane, and midazolam among others, it allows for measurement of the depth of anesthesia with these anesthetics [24], and is useful for measuring the degree of awareness after general anesthesia [25]. Thus, different from the previous study where only the recovery parameters were examined, we were able to obtain more objective information about the recovery by also measuring the BIS value.
In conclusion, this study showed that injection of flumazenil to unpremedicated patients anesthetized with sevoflurane/fentanyl could help in recovery from the anesthesia. This effect may be because flumazenil antagonizes the action of sevoflurane or intrinsic benzodiazepines. However, a large-scale study to verify our findings may be required to more accurately determine the effect and safety of flumazenil on recovery from general anesthesia.
References
1. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002; 2:833–839. PMID: 12171574.

3. Amrein R, Hetzel W, Hartmann D, Lorscheid T. Clinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988; 2:65–80. PMID: 2842143.
4. Philip BK, Simpson TH, Hauch MA, Mallampati SR. Flumazenil reverses sedation after midazolam-induced general anesthesia in ambulatory surgery patients. Anesth Analg. 1990; 71:371–376. PMID: 2119152.

5. Dahaba AA, Bornemann H, Rehak PH, Wang G, Wu XM, Metzler H. Effect of flumazenil on bispectral index monitoring in unpremedicated patients. Anesthesiology. 2009; 110:1036–1040. PMID: 19352163.

6. Karakosta A, Andreotti B, Chapsa C, Pouliou A, Anastasiou E. Flumazenil expedites recovery from sevoflurane/remifentanil anaesthesia when administered to healthy unpremedicated patients. Eur J Anaesthesiol. 2010; 27:955–959. PMID: 20864893.

7. Stucke AG, Zuperku EJ, Krolo M, Brandes IF, Hopp FA, Kampine JP, et al. Sevoflurane enhances gamma-aminobutyric acid type A receptor function and overall inhibition of inspiratory premotor neurons in a decerebrate dog model. Anesthesiology. 2005; 103:57–64. PMID: 15983457.
8. Salmi E, Kaisti KK, Metsähonkala L, Oikonen V, Aalto S, Någren K, et al. Sevoflurane and propofol increase 11C-flumazenil binding to gamma-aminobutyric acid A receptors in humans. Anesth Analg. 2004; 99:1420–1426. PMID: 15502041.
9. Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the γ-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology. 2003; 99:678–684. PMID: 12960553.
10. Kochs E, Wüst P, Blanc I, Schulte am Esch J. Acoustic and somatosensory evoked cortical potentials in sedation with midazolam and drug antagonism with flumazenil. Anasth Intensivther Notfallmed. 1989; 24:49–56. PMID: 2496619.
11. Weinbroum AA, Geller E. Flumazenil improves cognitive and neuromotor emergence and attenuates shivering after halothane-, enflurane- and isoflurane-based anesthesia. Can J Anaesth. 2001; 48:963–972. PMID: 11698314.

12. Roald OK, Forsman M, Steen PA. Partial reversal of the cerebral effects of isoflurane in the dog by the benzodiazepine antagonist flumazenil. Acta Anaesthesiol Scand. 1988; 32:209–212. PMID: 3129895.

13. Murayama T, Shingu K, Ogawa T, Tomoda K, Shindo K, Tamai S, et al. Flumazenil does not antagonize halothane, thiamylal or propofol anaesthesia in rats. Br J Anaesth. 1992; 69:61–64. PMID: 1637605.

14. Schwieger IM, Szlam F, Hug CC Jr. Absence of agonistic or antagonistic effect of flumazenil (Ro 15-1788) in dogs anesthetized with enflurane, isoflurane, or fentanyl-enflurane. Anesthesiology. 1989; 70:477–480. PMID: 2493754.

15. Schwartz AE, Maneksha FR, Kanchuger MS, Sidhu US, Poppers PJ. Flumazenil decreases the minimum alveolar concentration isoflurane in dogs. Anesthesiology. 1989; 70:764–766. PMID: 2497662.
16. Medina JH, Peña C, Piva M, Paladini AC, De Robertis E. Presence of benzodiazepine-like molecules in mammalian brain and milk. Biochem Biophys Res Commun. 1988; 152:534–539. PMID: 3365238.

17. Deckert J, Kuhn W, Przuntek H. Endogenous benzodiazepine ligands in human cerebrospinal fluid. Peptides. 1984; 5:641–644. PMID: 6089149.

18. Duthel JM, Constant H, Vallon JJ, Rochet T, Miachon S. Quantitation by gas chromatography with selected-ion monitoring mass spectrometry of "natural" diazepam, N-desmethyldiazepam and oxazepam in normal human serum. J Chromatogr. 1992; 579:85–91. PMID: 1447353.

19. Wildmann J, Niemann J, Matthaei H. Endogenous benzodiazepine receptor agonist in human and mammalian plasma. J Neural Transm. 1986; 66:151–160. PMID: 3023544.

20. Dencker SJ, Johansson G, Milsom I. Quantification of naturally occurring benzodiazepine-like substances in human breast milk. Psychopharmacology (Berl). 1992; 107:69–72. PMID: 1589563.

21. Ahboucha S, Pomier-Layrargues G, Butterworth RF. Increased brain concentrations of endogenous (non-benzodiazepine) GABA-A receptor ligands in human hepatic encephalopathy. Metab Brain Dis. 2004; 19:241–251. PMID: 15554420.

22. Sebel LE, Richardson JE, Singh SP, Bell SV, Jenkins A. Additive effects of sevoflurane and propofol on gamma-aminobutyric acid receptor function. Anesthesiology. 2006; 6. 104:1176–1183. PMID: 16732088.
23. Brogden RN, Goa KL. Flumazenil. A preliminary review of its benzodiazepine antagonist properties, intrinsic activity and therapeutic use. Drugs. 1988; 35:448–467. PMID: 2839329.
24. Sebel PS, Lang E, Rampil IJ, White PF, Cork R, Jopling M, et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesth Analg. 1997; 84:891–899. PMID: 9085977.

25. Flaishon R, Windsor A, Sigl J, Sebel PS. Recovery of consciousness after thiopental or propofol. Bispectral index and isolated forearm technique. Anesthesiology. 1997; 86:613–619. PMID: 9066327.
Fig. 1
Data are means ± SD. Two-way ANOVA with Bonferroni's posthoc test determined significant differences in BIS between the two groups from 6 to 12 min after flumazenil administration. *Indicates P < 0.05 between the groups. †Indicates P < 0.01 between the groups.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download