Abstract
Background
Elevated systemic levels of pro-inflammatory cytokines cause hypotension during septic shock and induce capillary leakage in acute lung injury. Manassantin B has anti-inflammatory and anti-plasmoidal properties. This study examined the effects of manassantin B on lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages.
Methods
RAW 264.7 macrophage cells were incubated without or with (1, 3 and 10 µM) manassantin B and without or with (100 ng/ml) LPS. Manassantin B dissolved in phosphate buffered saline was added to the medium 1 h prior to the addition of LPS. The degree of activation of mitogen-activated protein kinase (MAPK) including extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun amino terminal kinases (JNK) and p38 MAPK, and the level of interleukin (IL)-1β were determined 30 min and 24 h after the addition of LPS respectively.
Despite newly developed resuscitation therapies and the use of modern antibiotics, sepsis is still a leading cause of death in critically ill patients [1]. The mortality rate in sepsis patients remains as high as 40-70% [2]. Severe sepsis is characterized by hypoperfusion of major organs, leading to multiple organ failure, shock, and death. Inflammation, a physiological response to infection or injury, plays a key role in both health and disease. Several pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-8 promote systemic inflammation. Cytokine-mediated host defense mechanisms induce significant cell and organ injury and play crucial roles in the pathophysiology of sepsis [3].
IL-1β is produced from the cleavage of the inactive pro-IL-1β by stimulated leukocytes and is a fundamental contributor to local and systemic inflammatory responses [4]. IL-1β is a pro-inflammatory cytokine that functions as a critical regulator of the host defense in response to an infection or injury. However, IL-1β is extremely toxic when present in excess [5]. Elevated systemic levels of IL-1β may cause hypotension during septic shock and induce capillary leakage in acute lung injury [6]. IL-1β is also involved in chronic inflammation associated with arthritis, lung fibrosis and atherosclerosis [7-9]. Therefore, strategies to modulate IL-1β production in inflammatory diseases are of therapeutic interest. In addition, IL-1β activates the mitogen-activated protein kinase (MAPK) family including the extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 MAPK [10].
Nontoxic molecules that modulate macrophage-mediated inflammatory responses may provide a novel therapeutic strategy for the treatment of sepsis. Numerous studies have been performed to identify the new molecules. The manassantin group of structurally related and functionally unique dineolignans were recently isolated from active root extracts of Saururus cernuus L (Saururaceae), also referred to as "lizard tail" [11]. This fragrant aquatic plant is found in the North America as well as in many parts of Asia (Saururus chinensis) [12-14]. Although their structures and relative stereochemistries were only determined recently, the medicinal value of the plant has long been recognized by Native Americans, early colonists and practitioners of Korean traditional medicine for a range of diseases [14,15]. Manassantin A and B have biological activities, such as anti-inflammatory and anti-plasmodial effects [12,16].
The purpose of this study was to investigate the effect of manassantin B on LPS-induced activation of the pro-inflammatory cytokine IL-1β and intracellular signaling pathway such as the MAPKs signaling system, and the possibility that manassantin B might be a promising agent for reducing inflammatory response.
Manassantin B was donated from the College of Pharmacy, Yeungnam University, Korea. Escherichia coli 055:B5 endotoxin (lipopolysaccharide, LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies specific for phosphorylated (P)-ERK, P-JNK and p38 MAPK as well as total (T)-ERK, T-JNK and T-p38 MAPK were supplied by Cell Signaling Technologies (Beverly, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-1β were purchased from R&D Systems (Minneapolis, MN, USA). Dulbecco's modified Eagle's medium (DMEM) high glucose/L-glutamine, fetal bovine serum (FBS) and penicillin/streptomycin was obtained from GIBCO (Gaithersburg, MD, USA). Bicinchoninic acid (BCA) protein assay reagent was supplied by Pierce (Rockford, IL, USA).
The RAW 264.7 murine macrophage cell line was purchased from the Korea Cell Line Bank (Seoul, Korea). The cells were grown in DMEM supplemented with heat-inactivated 10% FBS, penicillin (100 U/ml) and streptomycin sulfate (100 µg/ml) in a 5% CO2 incubator at 37℃. The cells were plated at a density of 2 × 106 cells/well in a 6-well plate or 5 × 105 cells/well in 24-well plates. For all experiments, the cells were incubated until they reached 80-90% confluence. The adherent macrophages were washed gently with Ca2+ and Mg2+-free Dulbecco's phosphate buffered saline (DPBS) and cultured in serum-free DMEM for 2 h. Then, the cells were incubated in the absence or presence (1, 3 and 10 µM) manassantin B without or with LPS (100 ng/ml). Manassantin B dissolved in phosphate-buffered saline (PBS) was added to the medium 1 h prior to the addition of LPS. The following groups were then isolated in separate wells: control (manassantin B, 0 µM), manassantin B (1, 3 and 10 µM), LPS (100 ng/ml) and LPS+ manassantin B (1, 3 and 10 µM). The degree of activation of ERK1/2, JNK and p38 MAPK were determined at 30 min and the level of IL-1β at 24 h after the addition of LPS, respectively.
To evaluate the effect of manassantin B on the viability of RAW 264.7 macrophages, the cells were treated with various extract concentrations for 24 h, and then cell viability was evaluated using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. Twenty-four h prior to culture termination, 10 µl of the MTT solution (5 mg/ml in PBS, pH 7.4) was added to each well, and the cells were continuously cultured for 4 h. Then 100 µl of dimethyl sulfoxide (DMSO) was added for solubilization. Absorbance was measured at 570 nm using an ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each assay was repeated at least three times.
Immunoreactive IL-1β were quantified using commercially available ELISA kits (R & D Systems, Minneapolis, MN, USA), according to manufacturer's instructions as described previously [17].
Western blots to detect levels of phosphorylated and total ERK, JNK and p38 MAPK were performed essentially as previously described [18].
The detrimental effects of any compound or plant extract on the cell metabolism need to be determined before the biological activity can be examined. Cell viability was not significantly changed by the presence of manassantin B up to 10 µM (Fig. 1).
Manassantin B alone did not affect the production of IL-1β in RAW 264.7 cells over the range of concentrations examined. However, it attenuated an increase in IL-1β levels induced by LPS in a dose-dependent manner (Table 1).
The effects of manassantin B on the phosphorylation of ERK1/2, JNK and p38 MAPK were examined to determine its effect on MAPKs in LPS-stimulated RAW 264.7 cells. In a preliminary study, the maximum MAPKs phosphorylation occurred 30 min after the LPS (100 ng/ml) treatment in RAW 264.7 cells (data not shown). Therefore, the cells were incubated with manassantin B (1, 3, 10 uM) for 1 h and then with LPS (100 ng/ml) for 30 min. Manassantin B attenuated the phosphorylations of ERK1/2 and p38 MAPK in LPS-stimulated RAW 264.7 cells, but had no effect on JNK (Table 1, Fig. 2).
In this study, we investigated the effects of manassantin B, an active root extract of Saururus cernuus L, on proinflammatory cytokine production and activation of MAPKs in murine macrophage RAW 264.7 cells stimulated with LPS. Manassantin B attenuated the LPS-induced increase of inflammatory cytokine IL-1β and phosphorylations of signaling molecules ERK 1/2 and p38 MAPK. These findings suggest that manassantin B has an anti-inflammatory property.
RAW 264.7 murine macrophages are a popular macrophage cell model to examine a variety of inflammatory processes [19-21]. Macrophages are key regulators of inflammation and immunity [22]. Besides being involved in microbial clearance, they play an important role in regulating T cell function, proliferation, regulation of antibody production and production of mediators for cellular immunity. Many of these activities are mediated through pro-inflammatory cytokines [23]. When LPS is presented to Toll-like receptor-4 (TLR-4) in the cell membrane, an interaction via CD 14 and LPS binding protein leads to the activation of MAPKs, which are important for the LPS induced production of a range of inflammatory cytokines, including IL-1, IL-6, TNF-α and macrophage inflammatory protein (MIP)-2 [24]. These cytokines are essential for the eradication of pathogens. However, excessive release of inflammatory cytokines provokes life-threatening conditions, including sepsis, acute respiratory distress syndrome and multiple organ failure [25].
The MAPK signaling pathways consist of a series of kinases that are activated sequentially and consequently phosphorylate the downstream kinases and transduce extracellular stimuli into intracellular responses. The MAPK family includes ERK, JNK and p38 MAPK. One of the major functions of MAPK is the activation of transcription factors, several of which bind to the promoters of pro-inflammatory cytokines [26]. ERK1/2 regulates TNF-α and MIP-2 expression and ERK1/2 inhibitor (PD98059) reduces the expression of these cytokines [27,28]. Down-regulation of TLR 4 expression, using RNA interference techniques, decreases MAPKs activation, TNF-α and MIP-2 expression in RAW 264.7 cells stimulated with LPS [29]. In the present study, manassantin B treatment decreased the phosphorylation of ERK1/2 and p38 MAPK induced by LPS stimulation, but had no affect on JNK phosphorylation, suggesting that its anti-inflammatory activity is related to ERK1/2 and p38 MAPK cascades, but not JNK.
There are some limitations to this study. First, the study is limited in its in vitro character of the data, therefore much remains to be done in vivo studies in terms of safety and optimal dosage. Second, we measured only IL-1β as a pro-inflammatory cytokine. Potential interactions with other cytokines related with inflammation must be considered. Third, we measured the effects of manassantin B on the phosphorylation of MAPK without inhibitors due to the generalized results of inhibitor study.
In summary, manassantin B inhibits the production of the proinflammatory cytokine IL-1β and the phosphorylations of ERK 1/2 and p38 MAPK in LPS-stimulated RAW 264.7 murine macrophages. These findings suggest that manassantin B reduces LPS-induced IL-1β expression through effects on the ERK1/2- and p38 MAPK-mediated pathways in vitro. Thusm manassantin B might be a valuable therapeutic agent in reducing inflammatory response due to systemic inflammatory response syndrome involving sepsis in the clinical setting.
Acknowledgements
This study was supported by a grant (CRI09005-1) Chonnam National University Hospital Research Institute of Clinical Medicine.
References
1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303–1310. PMID: 11445675.

2. Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis--an overview. Acta Anaesthesiol Scand. 1999; 43:275–288. PMID: 10081533.
3. O'Reilly M, Newcomb DE, Remick D. Endotoxin, sepsis, and the primrose path. Shock. 1999; 12:411–420. PMID: 10588508.
4. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998; 30:1075–1079. PMID: 9785472.

5. Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993; 328:106–113. PMID: 8439348.

6. Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, et al. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res. 2008; 102:804–812. PMID: 18276918.
7. Fan Z, Söder S, Oehler S, Fundel K, Aigner T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007; 171:938–946. PMID: 17640966.

8. Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001; 107:1529–1536. PMID: 11413160.
9. Waehre T, Yndestad A, Smith C, Haug T, Tunheim SH, Gullestad L, et al. Increased expression of interleukin-1 in coronary artery disease with downregulatory effects of HMG-CoA reductase inhibitors. Circulation. 2004; 109:1966–1972. PMID: 15051633.

10. Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000; 43:2501–2512. PMID: 11083274.

11. Hanessian S, Reddy GJ, Chahal N. Total synthesis and stereochemical confirmation of manassantin A, B, and B1. Org Lett. 2006; 8:5477–5480. PMID: 17107051.

12. Hwang BY, Lee JH, Nam JB, Hong YS, Lee JJ. Lignans from Saururus chinensis inhibiting the transcription factor NF-kappaB. Phytochemistry. 2003; 64:765–771. PMID: 13679100.
13. Lee WS, Baek YI, Kim JR, Cho KH, Sok DE, Jeong TS. Antioxidant activities of a new lignan and a neolignan from Saururus chinensis. Bioorg Med Chem Lett. 2004; 14:5623–5628. PMID: 15482936.

14. Hartwell JL. Plants used against cancer. A survey. Lloydia. 1971; 34:204–255. PMID: 4938826.
15. Hussain RA, Lin YM, Poveda LJ, Bordas E, Chung BS, Pezzuto JM, et al. Plant-derived sweetening agents: saccharide and polyol constituents of some sweet-tasting plants. J Ethnopharmacol. 1990; 28:103–115. PMID: 2314108.

16. Kraft C, Jenett-Siems K, Köhler I, Tofern-Reblin B, Siems K, Bienzle U, et al. Antiplasmodial activity of sesquilignans and sesquineolignans from Bonamia spectabilis. Phytochemistry. 2002; 60:167–173. PMID: 12009320.

17. Kwak SH, Mitra S, Bdeir K, Strassheim D, Park JS, Kim JY, et al. The kringle domain of urokinase-type plasminogen activator potentiates LPS-induced neutrophil activation through interaction with {alpha}V{beta}3 integrins. J Leukoc Biol. 2005; 78:937–945. PMID: 16033814.
18. Bae HB, Li M, Kim JP, Kim SJ, Jeong CW, Lee HG, et al. The effect of epigallocatechin gallate on lipopolysaccharide-induced acute lung injury in a murine model. Inflammation. 2010; 33:82–91. PMID: 19838780.

19. Wong SS, Zhou HR, Marin-Martinez ML, Brooks K, Pestka JJ. Modulation of IL-1beta, IL-6 and TNF-alpha secretion and mRNA expression by the trichothecene vomitoxin in the RAW 264.7 murine macrophage cell line. Food Chem Toxicol. 1998; 36:409–419. PMID: 9662416.
20. Jung WJ, Sung MK. Effects of major dietary antioxidants on inflammatory markers of RAW 264.7 macrophages. Biofactors. 2004; 21:113–117. PMID: 15630180.

21. Schildknecht S, Heinz K, Daiber A, Hamacher J, Kavakli C, Ullrich V, et al. Autocatalytic tyrosine nitration of prostaglandin endoperoxide synthase-2 in LPS-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2006; 340:318–325. PMID: 16375865.

22. Solbach W, Moll H, Röllinghoff M. Lymphocytes play the music but the macrophage calls the tune. Immunol Today. 1991; 12:4–6. PMID: 2015046.

24. Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005; 2:20–27. PMID: 16212907.
25. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000; 342:1334–1349. PMID: 10793167.

26. Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J Immunol. 1999; 162:5367–5373. PMID: 10228013.
27. Shi L, Kishore R, McMullen MR, Nagy LE. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. Am J Physiol Cell Physiol. 2002; 282:C1205–C1211. PMID: 11997234.
28. Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994; 1:93–103. PMID: 8790605.

29. Xu Z, Huang CX, Li Y, Wang PZ, Ren GL, Chen CS, et al. Toll-like receptor 4 siRNA attenuates LPS-induced secretion of inflammatory cytokines and chemokines by macrophages. J Infect. 2007; 55:e1–e9. PMID: 17336389.

Fig. 1
Effect of manassantin B on the viability of RAW 264.7 macrophages. The cells were treated with various extract concentrations for 24 h, and the cell viability was measured using a methylthiazolyldiphenyl-tetrazolium bromide assay. The data are reported as mean ± SD (n = 4).
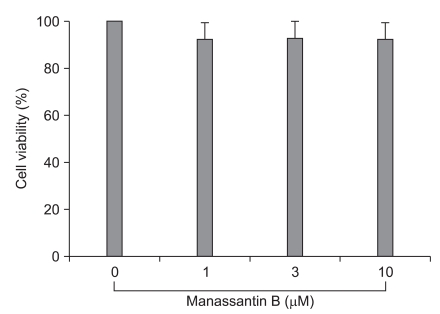
Fig. 2
Effects of manassantin B on LPS-induced ERK1/2, JNK and p38 MAPK phosphorylation in RAW 264.7 cells. The cells were incubated with media only (CON) or the indicated concentrations of manassantin B for 1 h and then incubated with LPS (100 ng/ml) for 30 min. P: phosphorylated, T: total.
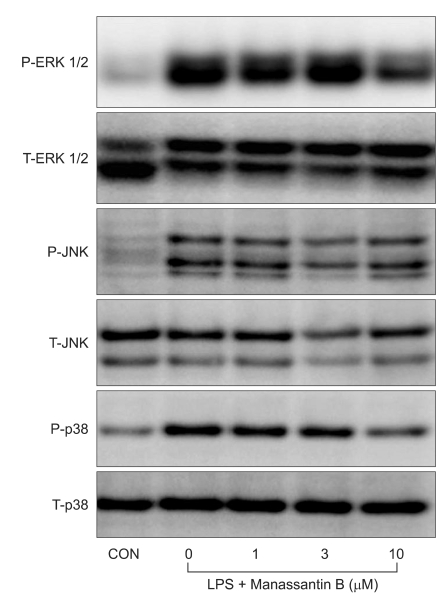
Table 1
The Effects of Manassantin B on LPS Induced IL-1β Production and MAPKs Phosphorylation in RAW 264.7 Cells
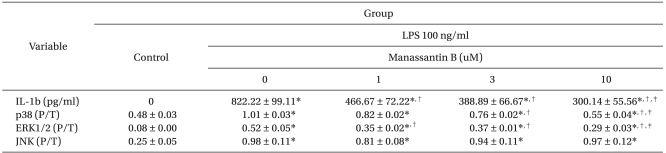
Each score represents mean ± SD. Control: The cells were incubated with manassantin B (0, 1, 3 and 10 µM) only for IL-1β and medium only for MAPKs. P/T: relative increase of phosphorylated- to total-MAPKs. *P < 0.05 versus control, †P < 0.05 versus LPS 100 ng/ml + manassantin B 0 uM, ‡P < 0.05 versus LPS 100 ng/ml + manassantin B 1 uM.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download