Abstract
Background
The purpose of this study was to evaluate the gender-related changes in the function and distribution of α1-adrenoceptors in the distal mesenteric artery of streptozotocin (STZ)-induced diabetic rats at the level of α1-adrenoceptor subtypes.
Methods
Diabetes was induced by intravenous injection of STZ in a dose of 60 mg/kg through the tail vein in 8 week-old male or female Sprague-Dawley rats (n = 13/group). Age-matched normal rats (n = 15) were used as a control group. Four weeks after STZ injection, the change in mean arterial pressure caused by a 45° tilting was recorded. The α1-adrenoceptor subtypes mediating contractions of the distal mesenteric artery were investigated using the agonist, phenylephrine as well as subtype-selective antagonists including prazocin, 5-methylurapidil, and BMY 7378. The expression of α1-adrenoceptor subtypes of each artery was examined by immunofluorescence staining and western blotting using subtype selective antibodies.
Results
Compared with normal male rats, the contractile response to phenylephrine was decreased in the distal mesenteric artery in normal female rats. Moreover, a decrease in contractile force was observed in STZ-induced diabetic rats compared with age-matched controls. Western blotting revealed that there was the difference between normal male and female rats in manifestation of the α1D-adrenoceptor. In STZ-induced male and female diabetic rats, all α1-adrenoceptor subtypes were decreased in distal mesenteric arteries, compared with normal rats.
There are increasing evidences that alterations induced by diabetes in the cardiovascular autonomic system may show gender differences [1]. Generally, cardiovascular diseases are more frequently found in men than premenopausal women [2]. However, in the diabetic patients, the incidence of cardiovascular disease becomes similar between men and women because diabetes may produce a relatively greater impairment in the female cardiovascular system [1]. In addition, in female rats, more severe diabetic-induced vascular impairment was developed than in male rats by impaired sympathetic mediated vasoconstriction or increase the relaxation to nitric oxide [3].
The activity of vascular sympathetic nervous system is regulated by adreneral hormone, several neurotransmitters and interaction with these factors via α1-, α2- and β-adrenoceptors [4]. In blood vessels, post-junctional α1-adrenoceptors play an important role in controlling vascular smooth muscle tone and thus modulate peripheral arterial resistance [5-7]. Radioligand binding, molecular cloning studies and isolated tissue experiments have identified three α1-adrenoceptor subtypes that are designated α1A, α1B, and α1D [8,9] and the functional dominance in adrenergic responses varies with tissue and species [10]. Considering these findings, diabetes-induced functional and distributional changes in the vascular α1-adrenoceptor can play a role in the pathogenesis of diabetic vascular disturbances, depending on the gender difference.
However, to our knowledge, there has been no investigation on how the α1-adrenoceptor subtypes change during the development of diabetic autonomic neuropathy depending on the gender. Therefore, we evaluated the effect of diabetes on function and distribution of the vascular α1-adrenoceptor using the distal mesenteric artery in streptozotocin (STZ)-induced diabetic male and female rats.
Twenty-eight male and female Sprague-Dawley rats (8 weeks old, 200-250 g) were used. All animal studies were carried out in a semi-pathogen-free barrier zone at Institute for Laboratory Animal Research in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals.
Male or female rats were randomly allocated into either the control group (n = 15) or diabetic group (n = 13). Diabetes was induced by a single intravenous tail vein injection of 60 mg/kg streptozotocin (STZ) dissolved in citrate buffer at pH 4.5. In the age-matched control groups, the same volume of citrate buffer was injected intravenously. All rats were housed in cages and allowed free access to food and water. Three days thereafter, blood glucose levels were measured from a drop of blood taken from the tail using OneTouch Ultra® Blood Glucose Meter (LifeScan, Inc). Rats with blood glucose >300 mg/dl were considered diabetic. All rats were maintained for 4 weeks without intervention.
Four weeks after STZ or citrate buffer injection, blood glucoses levels and body weights were measured. Body weights and blood glucose levels in normal and diabetic rats are shown in Table 1. Anesthesia was induced with sevoflurane-oxygen in a chamber and maintained with 2 vol% sevoflurane. Following anesthesia, rats were positioned in the supine position and four legs were firmly fixed on a tilt board. The tail artery was cannulated with a 24 G catheter for continuous arterial pressure monitoring. The transducer was positioned at the level of the heart. After measuring mean arterial pressure in the supine position (basal MAP), the table was vertically tilted at 45° angle in a head-up direction and MAP at 10, 30, 60, and 120 seconds after tilting were recorded. Changes in MAP related to tilting were compared at each time point between diabetic and control groups.
After the tilting experiment, the rat was euthanized by cervical dislocation under deep anesthesia and the third branch of mesenteric artery was dissected. Arteries were placed in a Petri dish with ice-cold modified Krebs' solution of the following composition (in mM): NaCl, 119; KCl, 4.7; MgSO4, 1.17; NaHCO3, 22; CaCl2, 1.6; HEPES, 8; KH2PO4, 1.18; d-glucose, 5. The artery was cleaned of fat and connective tissues under a microscope (Zeiss, Germany). To exclude the effect of endothelium-derived relaxing factor, the endothelium of the arteries was denuded by passing silk through the lumen. Thereafter, each artery was cut into 3 mm long rings for in vitro contractile studies. The remaining vessels were immediately stored at -80℃ for immunofluorescence staining and western blotting.
Each vascular segment, suspended between two triangle-shape tungsten hooks, was mounted in a 5 ml water-jacketed temperature-controlled tissue bath (Harvard Apparatus, Holliston, USA) containing modified Krebs' solution. The lower and upper hooks were fixed to the bottom of the bath chamber and to a force displacement transducer, respectively (NP 72-4493, Harvard Apparatus, Holliston, USA). The tissue bathing solution was equilibrated and suspended with a mixture of 95% O2 and 5% CO2 (pH: 7.38-7.42, pCO2: 34-36 mmHg) and maintained at 37℃. Propranolol (1 × 10-6 M) and yohimbine (1 × 10-7 M) were always included in the tissue bathing solution to block β- and α2-adrenoceptors, respectively. Changes in isometric force were recorded through an A/D converter (Powerlab®, ADInstruments, Colorado Springs, USA) and analyzed by using Chart for Window Software® (ADInstruments, Colorado Springs, USA). Following an initial tension of 2.0 g, which provided the optimum concentration-response relationships to KCl 60 mM, the arterial strips were allowed to equilibrate for 60 min. When the resting tension reached a stable baseline, no attempt was made to adjust tissue tension. After the equilibrium, tissues were primed by the addition of phenylephrine (1 × 10-6 M) to the organ bath. After a steady-state contraction was achieved, bath contents were replaced with modified Krebs' solution several times. Tissues were then allowed to reach baseline tension and the priming procedure was repeated once more before constructing concentration-response curves. This priming procedure reduces tissue variability in response to repeated agonist administration. In addition, the absence of functional endothelium was also confirmed by the loss of the relaxation response to acethylcholine (1 × 10-6 M) in the pre-contracted rings with a priming dose of phenylephrine (1 × 10-6 M) during the priming procedure.
Reproducible cumulative concentration-response curves were constructed by exposing each artery to increasing concentrations of phenlyephrine (1 × 10-9 to 1 × 10-4 M at 0.5 log unit increments) until a sustained maximum response (Emax) was observed. Concentration-response curves to phenylephrine were then obtained (each at 60 min apart) in the presence of three selective α1-adrenoceptor antagonists to each artery and the procedure was repeated with a high concentration of antagonists in the same preparation. The incubation time for selective α1-adrenoceptor antagonists, prazosin (α1; 1, 3, and 10 × 10-9 M), 5-methylurapidil (5-MU; α1A; 3, 10, and 30 × 10-8 M), and BMY 7378 (α1D; 3, 10, and 30 × 10-8 M) was 30 min. Propranolol (1 × 10-6 M) and yohimbine (1 × 10-7 M) were always included in the tissue bath solutions to block β - and α2-adrenoceptors, respectively.
After removing vessels and cleaning extraneous tissues, Hematoxylin and eosin (H&E) staining was used to assess the morphologic changes. Arteries were cut into 2-mm rings, embedded in OCT compound (Tissue-Tek® OCT™ Compound, Sakura Finetek, Japan) and quickly frozen (-50℃ in isopentane cooled with dry ice). Frozen blocks were cut into 5-µm sections and mounted on a slide. All sections were stored at -80℃ until use. After warming at room temperature for 30 minutes, the tissue sections were fixed in ice cold acetone for 5 minutes. After rinsing the sections in PBS, a blocking solution with 10% normal donkey serum was placed on the sections and then incubated for 30 minutes to block non-specific binding sites of immunoglobulin. After removal of the blocking solution, the tissue section was incubated with 1 : 100-diluted the primary antibody (α1D-AR cat. no. s.c.-1475, α1A-AR cat. no. s.c.-1477; Santa Cruz Biotechnology, Santa Cruz, USA) for 60 minutes at room temperature. After washing the primary antibody in PBS, fluoresceinisothiocyanate (FITC)-conjugated donkey anti-goat IgG (1 : 100 dilution, no. s.c.-2024; Santa Cruz Biotechnology) secondary antibody was placed on the sections for 60 minutes in the dark at room temperature. After removing secondary antibody by washing with PBS, mounting media was placed on the slide and a glass coverslip was adhered. To reduce the technical error, staining was performed on as same slide for each type of artery from each group. Three independent investigators observed the expression of α1-adrenoceptor subtypes of each artery with a fluorescence microscope (Olympus VENOX-AHBT3, Tokyo, Japan).
Isolated vascular segments were homogenized in lysis buffer (1% Nonidet-40, 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM Ethylenediaminetetracetic acid [EDTA], 0.1% SDS and 2 mM Phenylmethylsulfonyl fluoride [PMSF]). The sample was centrifuged at 12,000 g for 15 min at 4℃ and the supernatant was extracted. Protein concentrations were determined using the method of Sambrook and Russel [11]. Samples containing 180 ug of protein were boiled for 5 min to extract membrane proteins, loaded and electrophoresed on SDS-Polyacrolamide gels (PAGE), transferred to PVDF and then blocked for 1 h with 5% nonfat dry milk in tris-buffered saline with tween (TBST®; 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) for 1 hr at room temperature. Commercially available polyclonal antibodies to α1A- and α1D-adrenergic receptors (α1A-AR cat. no. s.c.-1477, α1D-AR cat. no. s.c.-1475; Santa Cruz Biotechnology) were applied to membranes (1 : 500 in TBST) overnight at 4℃. The membranes were washed with TBST® and secondary antibody diluted to 1 : 4,000 (Horse-radish peroxidase conjugated donkey anti-gout IgG; no. s.c.-2020; Santa Cruz Biotechnology) was incubated in room temperature with the membranes. After washing with TBST®, binding of the antibodies was detected with Chemiluminescent (ECL®, Amersham, Little Chalfont, Buckinghamshire, UK).
Data are presented as means ± SD, means ± SEM, number of rats, or number of arterial segments. In each concentration-response curve, the changes in tension related to increasing concentrations of phenylephrine are expressed as the difference of tension (g) between the basal values and the measured value with various concentrations of phenylephrine. Curves were fitted to all the data by non-linear regression using GraphPad Prism software (GraphPAD software, San Diego, USA) to calculate the negative logarithm of the concentration of agonist that provokes a half-maximal response (pEC50 = agonist potency). A pA2 value and Schild slopes were calculated by the method of Arunlakshana and Schild [12]. Using three different concentrations of each antagonist, the ratios between the half-maximal concentrations (EC50) of phenylephrine obtained with the specific antagonist concentration and that obtained in its absence (concentration ratio, R) were calculated when the maximal amplitudes between concentration-response curves in those two conditions were similar. Data were plotted as negative log-concentrations of the antagonist on the x-axis vs. log (R-1) on y-axis and fitted as a regression line. For calculation purposes, the slope parameter was set to -1.0 when statistically not different from unity. The x-intercept of the fitted regression line was an estimated pA2. The value of pA2 presented a logarithmic measurement of the potency of the specific antagonist and also the dose of antagonist that required a 2-fold increase in agonist concentration. Data were compared by two-way ANOVA, followed by unpaired Student t test with the Bonferroni correction for multiple comparisons, to determine which comparisons were statistically significant. Data were considered significantly different at values of P < 0.05.
Diabetic rats showed significantly higher blood glucose concentrations and lower body weight than age-matched normal rats (Table 1, all P < 0.001). After a 45° tilting in the head-up direction, recovery of MAP was significantly slower in diabetic rats than in normal rats and recovery in normal female rats were significantly slower than in normal male rats (Fig. 1, all P < 0.05).
The concentration-response curves for phenylephrine-induced contraction of normal mesenteric artery showed that the Emax and -logEC50 in female rats were significantly lower than those in male rats (Table 2). Diabetic rats had the significantly lower Emax and -logEC50 than normal rats. Further, the Emax in diabetic female group was lower than that in diabetic male group but there was no significant difference in the -logEC50 (Table 2).
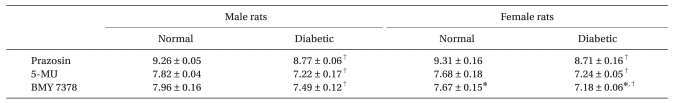
Regardless of arterial type, prazocin, 5-MU, and BMY 7378 shifted the concentration-response curve according to phenylephrine to the right in both diabetic and normal rats. In addition, pA2 values for prazocin, 5-MU, and BMY 7378 for inhibiting the contractile response to phenylephrine decreased by a similar order of magnitude in distal mesenteric arteries from diabetic rats (all P < 0.001, Table 3). In normal female rats, the pA2 value for BMY 7378 was significantly smaller than in normal male rats.
H & E staining images in male and female mesenteric arteries from normal and streptozotocin-induced diabetic rats showed that diabetic mesenteric arteries were characterized by a decrease in density of muscle fiber at the media of the vessel wall (Fig. 2). Immunofluorescence images of α1A- and α1D-adrenoceptor subtypes in mesenteric arteries of diabetic rats were characterized by a decreased fluorescent intensity (Fig. 3 and 4).
The α1D-adrenoceptor subtype was expressed less in female normal rats than in male normal rats (Fig. 5). In diabetic rats, the expressions of the α1A- and α1D-adrenoceptor subtypes were decreased compared with those in the gender-matched normal rats (all P < 0.05). In particular, in female diabetic rats, the expression of alpha1D - receptors was not detectable above background.
The results of our study showed that diabetes impaired the MAP recovery after tilting irrespective of gender and the MAP changes after tilting were different between normal male and female rats. The concentration-response curves for phenylephrine-induced contraction of normal female mesenteric artery showed that the adrenergic-mediated vasocontraction was impaired via difference in α1D-adrenoceptor expression. In diabetic rats, the adrenergic mediated vasocontraction was decreased irrespective of gender, which might be caused by the general decrease in overall α1-adrenoceptor subtype expression.
Vascular α1-adrenoceptor plays a primary role in maintaining systemic arterial pressure and arteriolar resistance [5-7]. Recent studies have shown that the α1D-adrenoceptor plays a major role in vasoconstriction of mesenteric arteries [13,14] and that the α1A-adrenoceptor is responsible for vascular contraction in distal mesenteric arteries [13]. A number of studies have demonstrated vascular differences depending of gender. Nitric oxide release which is an important endothelium-derived vasodilator regulating the vasomotor tone [15] depends on circulating estradiol concentrations [16]. In addition, estrogen decreases resistance artery adrenergic sensitivity by increasing the basal release of relaxing factors from the endothelium [17]. Our results in normal rats were comparable to a previous study [18] in which that the contractile responses to phenylephrine were significantly increased by endothelium disruption in arteries in males but not females. A possible explanation is that there was a significant difference in the pA2 for the α1D-adrenoceptor and normal female rats showed a relatively suppressed manifestation of α1D-adrenoceptor than normal males in immunofluorescence and western blotting. Similar to our results, the expression of α1D - adrenergic receptors was significantly suppressed by estradiol replacement [19].
Diabetic male or female rats had a 15% lower Emax and 5 or 2 times higher EC50 for phenylephrine than did the controls. These were in close accordance with our immunofluorescence staining and western blotting results. There were some discrepancies about the adrenergic responses of the mesenteric arteries of STZ-rats. The conflicting results could be due to differences in the vascular responses between the superior mesenteric arteries and small mesenteric arteries as well as the intact mesenteric bed, where the latter more reflects the resistance arteries [20]. In addition, diabetic female mesenteric arteries had a 20% lower Emax than diabetic male. These results were similar with previous investigations in which contractile responses to phenylnephrine were higher in diabetic males than in female rats [21] and the sensitivity to adrenergic nerve stimulation was greater in males compared to females [22].
Diabetic rats showed impaired recovery from the tilt-induced decrease in blood pressure. This phenomenon is very comparable with orthostatic hypotension. Considering that orthostatic hypotension is the most serious clinical consequence of diabetes-induced vascular disturbances [23], our diabetic rat model would be appropriate for evaluating the effect of diabetes on the vascular alpha 1-adrenoceptor. In normal female rats, the MAP changes after tilting were significantly smaller than those in the other groups when assessed at 10 sec to 120 sec after tilting. On the contrary, most clinical studies report that the orthostatic hypotension in women occurs more frequently than normal men [24-26]. However, the smaller degree of MAP changes is in women [27,28] and only changes in systolic BP to stimuli has gender differences [29]. A possible explanation is that the effects of α2 and β-adrenoceptors cannot be ruled out in vivo because the activity of vascular sympathetic nervous system is regulated by interaction via α1-, α2- and β-adrenoceptors [4].
Our study has some limitations. First, the α1A and α1D subtypes were chosen on the basis of their physiological roles in vivo in this study. It is reported that the contractions of the rat mesenteric artery are mediated via the α1D and α1A-subtypes which werewas identified as the predominant subtypes in rat mesenteric arteries [13,14]. In contrast, it appears that the α1B-subtype does not contribute to phenylephrine-induced pressor responses, nor may it be important for the maintenance of basal mean arterial pressure [30]. Second, 2 vol% sevoflurane may not result an equipotent depth of anesthesia between normal and diabetic rats when assessed in the tilting test. In diabetic rats, the decrease of vascular resistance by sevoflurane may be potentiated due to diabetes, which may make the difference greater.
In conclusion, the adrenergic-mediated vasocontraction was impaired via the decrease of α1D-adrenoceptors in normal female mesenteric arteries. Progression of diabetes decreased the contractile response in mesenteric arteries in STZ-induced diabetic rats irrespective of gender, which might be caused by the overall change in α1-adrenoceptors and not from the change in specific α1-adrenoceptor subtypes. Our results on the gender differences may be helpful in understanding one of the complex pathophysiologic changes in vascular disturbance caused by diabetes.
References
1. Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res. 2002; 53:678–687. PMID: 11861039.

2. Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000; 46:28–49. PMID: 10727651.

3. Farmer JA, Gotto AM. Dyslipidemia and other risk factors for coronary artery disease. A Textbook of Cardiovascular Medicine. 1997. 5th ed. Philadelphia: W.B. Saunders;p. 1126–1157.
4. van Zwieten PA. Antihypertensive drugs interacting with alpha- and beta-adrenoceptors. A review of basic pharmacology. Drugs. 1988; 35(Suppl 6):6–19. PMID: 2841084.
5. Ruffolo RR Jr, Hieble JP, Brooks DP, Feuerstein GZ, Nichols AJ. Drug receptors and control of the cardiovascular system: recent advances. Prog Drug Res. 1991; 36:117–360. PMID: 1876708.

6. Langer SZ, Schoemaker H. Alpha-adrenoceptor subtypes in blood vessels: physiology and pharmacology. Clin Exp Hypertens A. 1989; 11(Suppl 1):21–30. PMID: 2545380.

7. Jarajapu YP, Coats P, McGrath JC, Hillier C, MacDonald A. Functional characterization of alpha(1)-adrenoceptor subtypes in human skeletal muscle resistance arteries. Br J Pharmacol. 2001; 133:679–686. PMID: 11429392.
8. Graham RM, Perez DM, Hwa J, Piascik MT. Alpha 1-adrenergic receptor subtypes. Molecular structure, function, and signaling. Circ Res. 1996; 78:737–749. PMID: 8620593.
9. Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995; 47:267–270. PMID: 7568329.
10. Civantos Calzada B, Aleixandre de Artinano A. Alpha-adrenoceptor subtypes. Pharmacol Res. 2001; 44:195–208. PMID: 11529686.

11. Sambrook J, Russel DW. In vitro amplification of DNA by the polymerase chain reaction. Molecular Cloning. 2001. 3rd ed. New York: Cold Springs Harbor Laboratory Press;p. 18.
12. Schild HO. pA, a new scale for the measurement of drug antagonism. Br J Pharmacol Chemother. 1947; 2:189–206. PMID: 20258355.

13. Marti D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, et al. Correlation between mRNA levels and functional role of alpha1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the alpha1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 2005; 289:H1923–H1932. PMID: 15951348.
14. Arevalo-Leon LE, Gallardo-Ortiz IA, Urquiza-Marin H, Villalobos-Molina R. Evidence for the role of alpha1D- and alpha1A-adrenoceptors in contraction of the rat mesenteric artery. Vascul Pharmacol. 2003; 40:91–96. PMID: 12646397.
15. McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002; 33:1685–1691. PMID: 12053012.
16. Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci U S A. 1992; 89:11259–11263. PMID: 1454805.

17. Meyer MC, Cummings K, Osol G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. The American journal of physiology. 1997; 272:H2264–H2270. PMID: 9176294.

18. McKee AP, Van Riper DA, Davison CA, Singer HA. Gender-dependent modulation of alpha 1-adrenergic responses in rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2003; 284:H1737–H1743. PMID: 12679329.
19. Zhang Y, Davidge ST. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension. 1999; 34:1117–1122. PMID: 10567192.

20. Brondum E, Nilsson H, Aalkjaer C. Functional abnormalities in isolated arteries from Goto-Kakizaki and streptozotocin-treated diabetic rat models. Horm Metab Res. 2005; 37(Suppl 1):56–60. PMID: 15918112.

21. Stallone JN. Sex differences in nitric oxide-mediated attenuation of vascular reactivity to vasopressin are abolished by gonadectomy. Eur J Pharmacol. 1994; 259:273–283. PMID: 7982454.

22. Li Z, Duckles SP. Influence of gender on vascular reactivity in the rat. J Pharmacol Exp Ther. 1994; 268:1426–1431. PMID: 8138955.
23. Watkins PJ, Thomas PK. Diabetes mellitus and the nervous system. J Neurol Neurosurg Psychiatry. 1998; 65:620–632. PMID: 9810929.

24. Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998; 275:R1909–R1920. PMID: 9843880.
25. Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol. 1994; 77:1776–1783. PMID: 7836199.
26. Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med. 1977; 48:138–145. PMID: 871283.
27. Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001; 281:H2028–H2035. PMID: 11668064.
28. Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005; 111:494–498. PMID: 15687139.

29. Light KC, Turner JR, Hinderliter AL, Sherwood A. Race and gender comparisons: I. Hemodynamic responses to a series of stressors. Health Psychol. 1993; 12:354–365. PMID: 8223359.

30. Patane MA, DiPardo RM, Price RP, Chang RS, Ransom RW, O'Malley SS, et al. Selective alpha-1a adrenergic receptor antagonists. Effects of pharmacophore regio- and stereochemistry on potency and selectivity. Bioorg Med Chem Lett. 1998; 8:2495–2500. PMID: 9873568.
Fig. 1
Time course for the change in mean arterial pressure (MAP) after tilting in normal male (▪), normal female (▴), streptozotocin-induced diabetic male (□) and diabetic female (▵) rats. Each data point represents means ± SD. Data from 13 rats were collected in each group. *P < 0.001 vs. diabetic rats. †P < 0.05 vs. normal rats, ‡P < 0.01 vs. male rats.
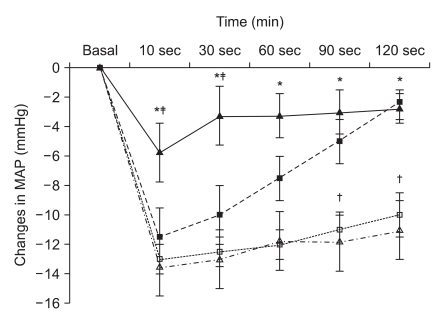
Fig. 2
Hematoxylin and eosin stained images in male (A, B) and female mesenteric arteries (C, D) from normal and streptozotocin-induced diabetic rats. (magnification: ×200). Mesenteric ar ter ies f rom diabet ic rat s were characterized by a decrease in density of muscle fiber at the media of the vessel wall.
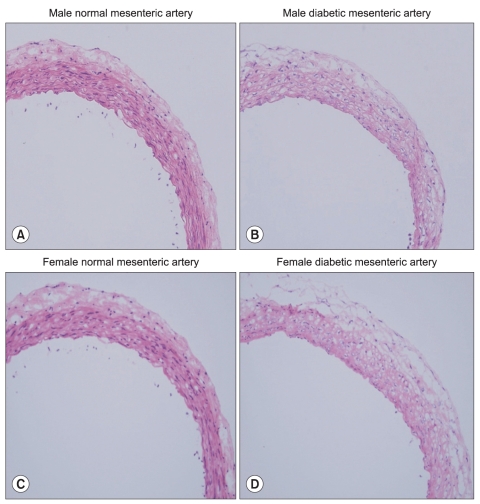
Fig. 3
Immunofluorescence images of the α1A-adrenoceptor subtype in male (A, B) and female mesenteric arteries (C, D) from normal and streptozotocin-induced diabetic rats. (magnification: ×200). Diabetic mesenteric arteries were characterized by a decreased fluorescent intensity.
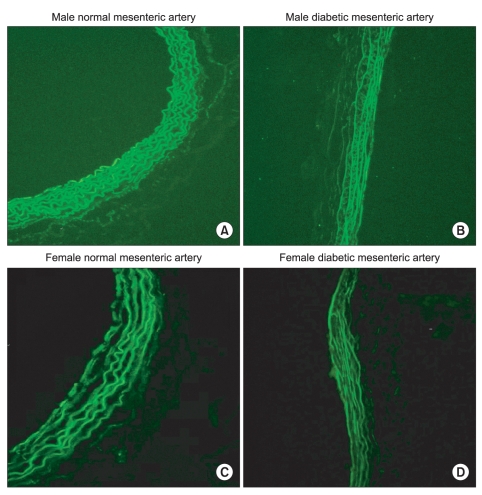
Fig. 4
Immunofluorescence images of the α1D-adrenoceptor subtype in male (A, B) and female mesenteric arteries (C, D) from normal and streptozotocin-induced diabetic rats. (magnification: ×200). Diabetic mesenteric arteries were characterized by a decreased fluorescent intensity.
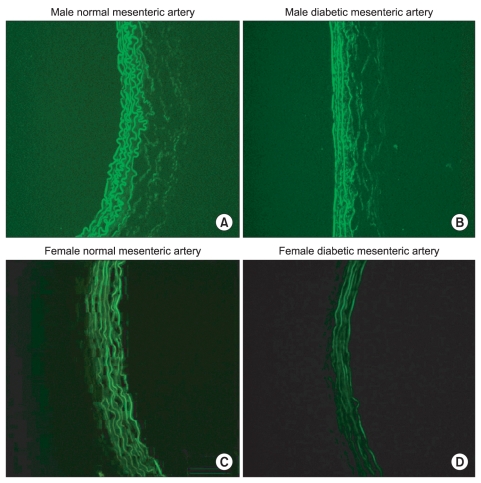
Fig. 5
(A) A representative Western blot was shown in the top panel and the graphic presentation of alpha 1 - receptors quantified by integrating the volume of autoradiograms from three separate experiments is shown in the bottom panel. (B, C) In diabetic rats, the expressions of the alpha 1A and alpha 1D - receptors were suppressed compared with those in normal rats. (C) The expression of the alpha 1D - receptors was suppressed in female normal rats compared with those in male normal rats. *P < 0.05 vs. male rats. †P < 0.05 vs. normal rats.
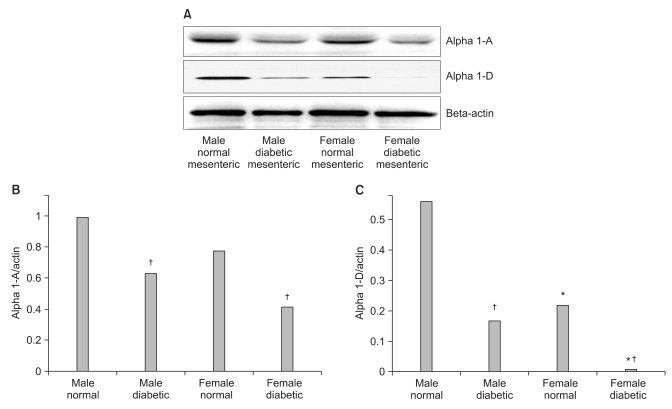
Table 2
Maximal Response (Emax) and -logEC50 Values for Phenylephrine-Induced Contraction of Distal Mesenteric Arteries from Normal and Streptozotocin-Induced Diabetic Rats





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download