This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This randomized, double-blinded clinical study was designed to evaluate the efficiency and safety of remifentanil with ketorolac for IV PCA after laparoscopic-assisted vaginal hysterectomy.
Methods
Eighty patients were randomly allocated into four groups. Group R received IV PCA using only remifentanil at a basal rate of 0.025 µg/kg/min and a bolus of 0.375 µg/kg. Group RK1 received IV PCA using remifentanil at a basal rate of 0.015 µg/kg/min and a bolus of 0.225 µg/kg. Group RK2 received IV PCA using remifentanil at a basal rate of 0.0075 µg/kg/min and a bolus of 0.1125 µg/kg. Group F received IV PCA using fentanyl at a basal rate of 0.3 µg/kg/h and a bolus of 0.075 µg/kg. In addition, ketorolac at a basal rate of 0.04 mg/kg/h and a bolus of 0.01 mg/kg was added to Group RK1, RK2, and F. All PCA conditions had a lock out period of 15 minutes. Pulse rate, systolic and diastolic BP, sedation score, visual analogue scale (VAS), and PONV score were recorded at 1, 3, 6, 12, and 24 hours after the operation. Total opioid use and the patients' number for rescue analgesic drug were also collected.
Results
The groups did not differ in PONV score and hemodynamic changes. The VAS in Group RK2 was high compared with the other groups. In addition, the sedation score was high in Group R.
Conclusions
The additional ketorolac administration in remifentanil IV PCA had remifentanil sparing effects and reduced sedation among the side effects. Further studies will be needed to evaluate the precise and adequate dosage of ketorolac.
Keywords: Ketorolac, Patient-controlled analgesia, Remifentanil
Introduction
Proper management of post-operative pain plays an important role in not only reducing distress caused by pain itself but also contributing to cardiovascular stability, improving respiratory function, and enabling early recovery [
1-
3]. A variety of methods for pain control has been introduced. Among them, intravenous patient-controlled analgesia (PCA) is well known for its excellent pain control and high stability minimizing the incidence of adverse effects. Commonly administered through PCA is combined anesthesia of different opioids or of opioid and nonsteroidal anti-inflammatory agents (NSAIDs).
The ideal opioid for PCA should have a rapid onset of action, produce no resistance and drug dependence, and minimize side effects without accumulation in the body [
4,
5]. Recently, the most commonly used opioid is morphine or fentanyl along with studies on other opioids such as sufentanil, alfentanil, and remifentanil [
6-
8]. Among them, remifentanil, an opioid of a potent, selective µ-opioid receptor agonist, is rapidly metabolized by non-specific esterases that are widespread throughout the plasma, red blood cells, and interstitial tissues, whereas other anilinopiperidine opioids depend upon hepatic biotransformation and renal excretion for elimination. By virtue of the consistently short context-sensitive half-life of remifentanil (3.2 minutes) regardless of infusion duration, it does not accumulate in the body in contrast to other opioids, and patients show fast recovery immediately after discontinuation of remifentanil [
9].
In addition, remifentanil has a rapid onset of peak effect and does not cause any critical side effects to the cardiovascular system. Because of these distinctive pharmacokinetic properties, remifentanil has been regarded as the ideal drug for post-operative analgesics and there have been various studies on pain control using remifentanil. However, some reports have revealed disadvantages of remifentanil and incidence of respiratory restraint such as apnea at administration of a large dose of remifentanil [
10,
11].
Ketorolac, one of the nonsteroidal anti-inflammatory agents (NSAIDs), if combined with an opioid through PCA, will enhance analgesic effects and lessen the requirement of the opioid dose, which eventually reduces several opioid-induced adverse effects [
12,
13]. It is noted, however, that studies on the opioid sparing effects of NSAIDs have been limited to conventional opioids such as morphine, fentanyl, etc. [
14,
15] and that there has been no studies on the combined treatment with remifentanil. If the opioid sparing effect of NSAIDs can be applied to remifentanil as in the other opioids, the reduction of the remifentanil requirement in treatment is expected to enable safer anesthesia with less over-dose side effects.
Therefore, the purpose of the present study was to investigate the remifentanil sparing effect in the combination therapy of ketorolac and remifentanil and to study the sedation effect or adverse reaction reducing effect on nausea and vomiting generated by the reduction of the opioid in patients who had undergone laparoscopic-assisted vaginal hysterectomy (LAVH), referring to the previous studies on administered dosages of remifentanil. In addition, we tried to determine the optimum concentration of remifentanil, which shows similar analgesic effects and similar occurrence rate of side effects to those of fentanyl intravenous (IV) PCA, commonly used with ketorolac recently.
Materials and Methods
The present study was approved by our Institutional Bioethics Board of clinical research, and the study population consisted of eighty patients with American Society of Anesthesiologists physical status I-II, who were scheduled to undergo laparoscopic-assisted vaginal hysterectomy (LAVH) under general anesthesia. Patients who had a medical history of mental disorders or drug abuse, who had liver or kidney disease, who were allergic to opioids or NSAIDs, and who were over the age of 65 years were excluded. On the day before the surgery, written informed consents were obtained after the purpose of this study, how to use the PCA pump, (verbal or numerical) pain rating scale scores, and any possible side effects were explained to the patients.
The eighty patients were randomly allocated into four groups of 20 patients each by block randomization. Patients in Group R were given only remifentanil (Ultiva®, GlaxoSmithKline, UK) with a basal rate of 0.025 µg/kg/min and a bolus dose of 0.375 µg/kg; Group RK1 and RK2 patients were given remifentanil with a basal rate of 0.015 µg/kg/min and 0.0075 µg/kg/min and a bolus dose of 0.225 µg/kg and 0.1125 µg/kg, respectively; patients in Group F received fentanyl (Fentanyl citrate®, Myungmoon Pharm. Co., Korea) with a basal rate of 0.3 µg/kg/h and a bolus dose of 0.075 µg/kg. Additionally, ketorolac (keromin®, Hana Pharm. Co., Korea) were given at a basal rate of 0.04 µg/kg/h and a bolus dose of 0.01 µg/kg to Group RK1, RK2, and F, respectively, with a 15-minute lockout time for all groups.
Before arrival into the operating room (OR), patients were premedicated with 0.2 mg of intramuscular glycopyrrolate. After arrival into OR, anesthesia was induced with 2 mg/kg of propofol. Rocuronium (0.6 mg/kg) was given to facilitate endotracheal intubation. Anesthesia was maintained with desflurane and 50% N2O. For proper muscle relaxation, 2 mg of vecuronium was administered one hour after anesthesia induction. At the completion of surgery, 0.4 mg/kg of glycopyrrolate and 10 mg/kg of pyridostigmine were administered to reverse residual curarization [muscle relaxation] and then the patients were transferred to the post-anesthesia care unit (PACU).
In the recovery unit, clinical data such as blood pressure (BP), heart rate, pulse, and oxygen saturation (SaO2) were collected, and the patients were prompted to use the IV PCA pump (accumate 3000, Woo Young Medical Co., Korea) for self-administering their own pain relief with a pre-programmed dosage for each group. For a loading dose, the patients in Group R, RK1, and RK2 received 1 µg/kg, 0.6 µg/kg, and 0.3 µg/kg of remifentanil, respectively, and Group F patients received 1 µg/kg of fentanyl. Except for Group R, all the patients were administered ketorolac with a loading dose of 30 mg. Assessment of pain, nausea, and sedation scale was made 1 hour, 3 hours, 6 hours, 12 hours, and 24 hours after initiation of IV PCA through interview with the patients. In addition, BP, pulse, and administration of other analgesics were recorded. Pain intensity was assessed by 0 to 10 numeric pain scores of visual analogue scales (VAS) at rest with 0 = no pain and 10 = the most severe pain, while the degrees of sedation and nausea/vomiting were evaluated separately in the following ways.
Sedation Scores
0. Awake and alert
1. Drowsy and responds appropriately
2. Sleep, arouse to verbal stimuli
3. Deep sleep, arouse to physical stimuli
4. Unarousable
Postoperative Nausea and Vomiting (PONV) scores
If patients complained of pain not relieved by the use of the PCA, meperidine 25 mg was given by intramuscular injection. When unbearable nausea happened or patients vomited, 10 mg of metoclopramide was administered. All data were presented as mean ± SD (standard deviation) and statistical analyses were done using SPSS 15.0 for Windows (SPSS Inc, Chicago, Illinois, USA). For VAS and sedation scores, BP, and pulse rates, repeated measure analysis of variance (ANOVA) were done to test the between-group factor and measurement changes within a group, followed by Mauchly's test of sphericity, tests of within-subjects effect and between-subjects effect, and Duncan's test as post-hoc test. In addition, for statistical comparison between the four groups, ANOVA test and Duncan's test as a post-hoc test were used on height, weight, age, duration of surgery, duration of anesthesia, and 24-hour dosage of opioid administered to Group R, RK1, and RK2. The incidence of nausea or vomiting per group was verified by one-way ANOVA test, followed by a multiple comparison test as a post-hoc test using Fisher's Least Significant Difference (LSD) test. For comparison of incidences using antiemetics or additional analgesics between the groups, Fisher's exact test was done. In all of the statistical analyses, P values < 0.05 were considered statistically significant.
Results
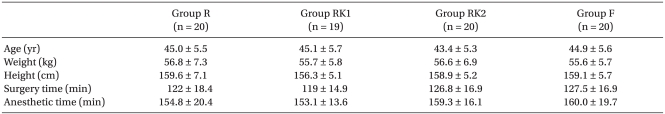
One patient in Group RK1 had to be excluded because of setting-up errors in the infusion pump apparatus for PCA. Afterward, this study proceeded with a total enrollment of 79 patients. There were no statistically significant differences in age, height, weight, and duration of surgery between the four groups (
Table 1).
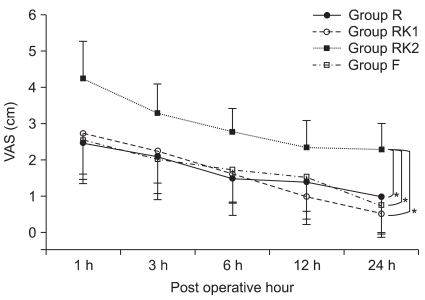
VAS scores decreased significantly over time for all of the four groups when IV PCA was started (P = 0.00), among which Group RK2 showed significantly higher VAS scores compared with the other three groups (P < 0.05,
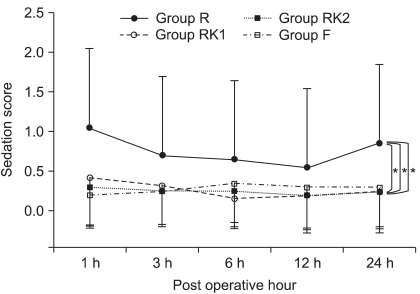
Fig. 1). For sedation scores, there was no significant difference between the groups in the changes over time, among which Group R showed significantly higher scores (P < 0.05,
Fig. 2).
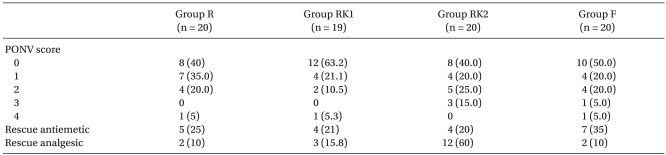
For the incidence of nausea or vomiting, the case of no nausea was dominant for all the groups, and there was also no significant difference between the groups, while the need for use of antiemetics was counted for 5 patients in Group R, 4 patients in Group RK1, 4 patients in Group RK2, and 7 patients in Group F, which did not make a statistical difference (
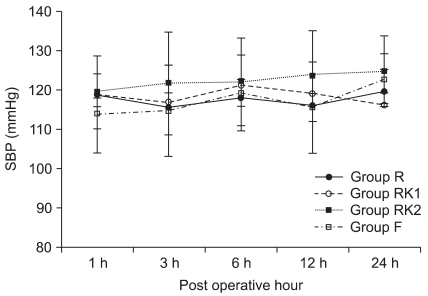
Table 2). There were no significant differences between the four groups in changes of systolic and diastolic BP, and heart rate during the course of PCA use (
Fig. 3,
4 and
5). In the number of patients taking additional dosages of analgesic, there were no significant differences between Group R, RK1, and F, whereas Group RK2 was significantly higher than the other three groups (
Table 2).
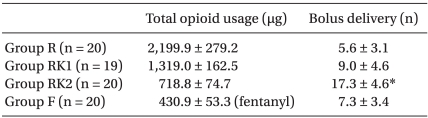
For the total quantities of opioid used for 24-hour period, there were significant differences in remifentanil use among the three groups: 2,199.9 ± 279.2 µg for Group R; 1,319.0 ± 162.5 µg for Group RK1; and 718.8 ± 74.7 µg for Group RK2 (P < 0.05,
Table 3), while 430.9 ± 53.3 µg of Fentanyl was used for Group F. Total bolus numbers were significantly higher in Group RK2 compared with the other three groups (P < 0.05,
Table 3).
Discussion
The authors proceeded with the present study under the hypothesis that additional ketorolac administration in remifentanil IV PCA, which has risks of excessive sedation or apnea, could be applied safely in clinical situations for postoperative pain control by virtue of the opioid sparing effects and reduced incidences of the above mentioned side effects due to a reduction in the total remifentanil demand. We have demonstrated that the combined use of 0.04 mg/kg/h of ketorolac resulted in a 0.01 µg/kg/min reduction of remifentanil and that the combination of 0.015 µg/kg/min of remifentanil and 0.04 mg/kg/h of ketorolac co-administered through IV PCA showed statistically similar analgesic effects but no difference in the incidence of side effects as with the existing fentanyl used IV PCA.
The sedative effects of remifentanil arise indirectly by inhibition of ascending cortical activation from the reticular activating system [
3]. Considering the common knowledge that respiratory depression or apnea may be caused by excessive sedation, it is such respiratory depression and apnea accompanied with excessive sedation that would be the biggest restriction on the use of remifentanil in IV PCA.
Kucukemre et al. [
16] reported that one patient suffered from respiratory failure after administering a loading dose of 45 µg in a PCA study using remifentanil and the patient required intervention with O2 (oxygenation) and ventilation support by mask. In a study conducted by Egan et al. [
17] to evaluate the safety of a single bolus dose of remifentanil, they observed that episodes of apnea occurred in 4 subjects receiving more than 75 µg.
Minto et al. [
18] maintained that the pharmacodynamic profile of remifentanil varies according to the age and lean body mass of the individual patients, while Egan et al. [
17] reported that at a bolus injection of remifentanil the incidence of respiratory depression and apnea increased gradually as the dosage of the bolus and the age of the patients increased. Due to such pharmacokinetic properties of remifentanil as narrow therapeutic index and susceptibility-induced wide individual variation [
3,
19,
20], it is difficult to identify the optimal dosage during pain control compared with other drugs and even small changes in concentration may endanger patients by excessive sedation or apnea suddenly.
Therefore, the current study set Group R as the basic experimental group in accordance with previous research results that IV remifentanil PCA at a basal rate of 0.025 µg/kg/min provided efficacious analgesia for patients undergoing laparoscopic-assisted vaginal hysterectomy (LAVH) [
21]. Since the potency ratio of remifentanil and ketorolac is not definitive in the combined analgesic, 0.015 µg/kg/min of remifentanil, 60% of the remifentanil used for Group R, was provided for Group RK1, while 0.0075 µg/kg/min of remifentanil, 30% of the remifentanil used for Group R, was provided for Group RK2. For ketorolac, we used a dosage generally recommended for administering ketorolac with other opioids in PCA [
12]. For Group F, the ketorolac and fentanyl IV PCA group that was set to compare the analgesic effects and the incidence of side effects with those of fentanyl IV PCA, the fentanyl basal rate was determined arbitrarily between 15 µg/h and 20 µg/h as usually done in administrating fentanyl with ketorolac after OB/GYN surgery.
Consequently, respiratory depression occurred in two patients in Group R after all of the load doses were administered. For one patient, a sedation event accompanied by loss of consciousness occurred after administration of a load dose of 60 µg. Afterwards, oxygen saturation dropped to below 90%. By administrating oxygen at 5 L/min using a mask, the patient was prompted to wakefulness and encouraged to breathe spontaneously. For the another patient, respiratory depression occurred after administering a load dose of 55 µg accompanied by the loss of consciousness with no response to prompting, cyanosis, and apnea, when 0.2 mg of naloxone was intravenously injected with O2 intervention and ventilation support. As soon as the two patients regained consciousness within 5 min, administration of medication through PCA was started. During a 1-hour monitoring period, when pain control was not satisfactory, the patients were encouraged to self-administer several bolus doses, while there was neither respiratory depression nor apnea requiring any medical attention. The authors reasoned that the events of respiratory depression for the two patients were caused by dose dependency oversedation rather than a hypersensitivity reaction to opioids because the infused dosage was similar to the bolus doses that induced respiratory depression in the previous studies. Ensuing continuous infusion with a bolus injection via IV PCA did not develop any further episodes such as respiratory depression. Then, we resumed the research procedure on the presumption that the determined PCA dose was adequate. For patients recovering from anesthesia, however, great care is necessary in administering a loading dose at which previous studies have anticipated the incidence of apnea because the presence of excess anesthetic gas might worsen sedation at the administration of an opioid. As in Group RK1 in the present study, it is advisable to reduce the loading dose of remifentanil by supplement administration of ketorolac or to replace remifentanil IV PCA by intramuscular (IM) injection of other opioids such as meperidine.
According to Parker et al. [
12], the opioid sparing effects of ketorolac decrease the respiratory depression effect caused by the administered opioids. Furthermore, Burns et al. [
22] and Bosek and Miguel [
23] reported that the use of ketorolac with opioids reduces opioid demand, which eventually decreases not only the incidences of hypoventilation, drug tolerance, and addiction but also the adverse effect of sedation. Also, in our study, which compared sedation scores measured in Group R and Group RK1, both of which showed statistically similar outcomes of pain control, Group RK1 revealed significantly lower scores. The result indicates that a combination of remifentanil and ketorolac reduces the adverse effects of remifentanil-induced sedation.
Although the quantity of the infused opioids was smaller in Group RK2 than in Group RK1, there was no significant difference in the sedation scores between the two groups. This seems to be because the number of patients receiving supplementary analgesics was higher in Group RK2 than in Group RK1, which offset the difference of total remifentanil quantity between the two groups. Since it is possible that the total quantity of infused opioids in the two groups would demonstrate the same sedation scores in reality, it is necessary to administer booster doses of other analgesics such as NSAIDs rather than opioids in order to make a precise comparison. Postoperative Nausea and Vomiting (PONV) scores did not show any statistically relevant differences between Group R, Group RK1, and Group RK2, which implies that use of ketorolac in the combination does not reduce the incidence of remifentanil-induced nausea or vomiting. In a study conducted by Kim et al. [
24] to investigate the fentanyl-sparing effect of ketorolac on patients who had undergone cesarean section, they reported that IV PCA combination of fentanyl and ketorolac developed less incidence of nausea than IV PCA with only fentanyl. However, they are not sure whether such a difference was derived from the fentanyl-sparing effect or from the difference between the two groups in pain, one of the nausea or vomiting inducers, when the group receiving fentanyl and ketorolac combination had more efficacious analgesic effects than the other group receiving only fentanyl. In addition, a previous study reported that the combination administration of ketorolac with other opioids such as morphine did not manifest a reduction of the side effects such as nausea, vomiting, and pruritus (itching) other than sedation [
14,
25,
26].
Comparing the analgesic effects of remifentanil and other established opioids on post-operative pain control, Dill-Russell et al. [
27] argued that remifentanil could be utilized as a good post-operative analgesic agent after observing that replacement of morphine by remifentanil in IV PCA decreased incidences of nightmare and nausea. In a study of IV PCA with morphine, fentanyl, and remifentanil after cardiac surgery, Gurbet et al. [
5] reported that the group receiving morphine showed a higher incidence of nausea and vomiting while the fentanyl group showed a significantly higher incidence of pruritus than the remifentanil group. A study done by Choi et al. [
28], where patients undergoing total hysterectomy were administered the minimum recommended (effective) analgesic concentration of fentanyl or remifentanil for post-operative pain control, has revealed that the analgesic effects and the incidence of PONV are similar in both groups, while remifentanil induces serious oversedation and respiratory depression. The present research results have demonstrated that Group F, the fentanyl IV PCA group administered at a basal rate of 0.3 µg/kg/h and Group RK1, the remifentanil IV PCA group administered at a basal rate of 0.015 µg/kg/min, both exhibited similar analgesic effects with similar VAS scores in the combination treatment using ketorolac, and that there was no statistically relevant difference in adverse effects between the groups. This concludes that the additional ketorolac administration in IV PCA administered with remifentanil at a basal rate of 0.015 µg/kg/min produces excellent effects on post-operative pain management in patients after LAVH surgery.
A cause of concern are some of the limitations of the present study such as the ratio of bolus delivery number to bolus demand number was not available to our knowledge due to the lack of bolus demand number function in the model of the PCA pump used, and that more different remifentanil dose settings were not available in circumstances lacking the exact potency ratio between remifentanil, ketorolac, and fentanyl. We also found that the bolus dose set for Group R merely amounted to 25% of the basal dose provided per hour, which kept interfering in setting test doses for other groups. As a result, patients' pain management became dependent almost on basal infusion and not enabling patients to control analgesic doses and administering intervals suitable for their pain in the course of time by pushing buttons, but being untrue to the name value of PCA (patient controlled analgesia). In conclusion, we have confirmed that the additional administration of ketorolac in remifentanil IV PCA for post-operative pain management can decrease adverse effects induced by narcotic analgesic overdose by virtue of reducing the remifentanil demand through the remifentanil-sparing effect.
Further studies are expected on a variety of remifentanil dose settings in the future.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download