Abstract
Background
The administration of short-acting opioids before emergence is useful for preventing emergence cough induced by an endotracheal tube. This study examined the clinically effective dose of alfentanil for suppressing cough during emergence from desflurane anesthesia.
Methods
Twenty-nine adult patients undergoing elective oral surgery were enrolled in this study. During emergence from anesthesia, the patients received alfentanil diluted in 10 ml normal saline when the end-tidal vol% of desflurane decreased to 3%. The initial alfentanil dose was 16 µg/kg. The alfentanil dose for consecutive patients, determined by Dixon's up-and-down method, increased or decreased by 2 µg/kg according to a previous patient's result.
Results
The 50% effective dose (ED50) of alfentanil for suppressing cough during emergence from desflurane anaesthesia was 9.3 ± 1.5 µg/kg according to Dixon's up-and-down method. Isotonic regression revealed an ED50 and ED95 (95% confidence interval) of alfentanil 10.0 µg/kg (6.8-13.2 µg/kg) and 14.0 µg/kg (7.7-19.4 µg/kg), respectively.
Endotracheal tube-induced coughing during emergence from general anesthesia is a common phenomenon with a reported incidence of 38-96% [1-3]. Emergence cough is associated with an increase in heart rate, blood pressure, intracranial, intraocular, and intra-abdominal pressure. These adverse effects can result in coronary ischemia, arrhythmia or wound dehiscence [4-6]. Several techniques or drugs have been investigated to prevent emergence cough, such as deep extubation [7], a change to laryngeal mask during emergence [8], topical or intracuff lidocaine application [9,10] and the administration of dexmedetomidine [11] or short-acting opioids [12-14]. The beneficial effects of short-acting opioids, such as alfentanil or remifentanil, for suppressing cough during emergence have been reported. However, there is no data on the optimal dose of alfentanil to prevent cough during emergence. This study examined the clinically effective single-dose of alfentanil for suppressing cough during emergence from desflurane anesthesia.
This study was approved by the institutional review board and written informed consent was obtained from the subjects. ASA I-II adult patients undergoing elective oral surgical procedures were enrolled in this study. The exclusion criteria included treatment with antitussives or angiotensin converting enzyme inhibitor, a history of chronic cough, asthma or recent respiratory tract infections. Obese patients (body mass index (BMI) >30 kg/m2) and patients with predicted difficult intubation were also excluded.
Thirty minutes before the induction of anesthesia, all patients were pre-medicated with intramuscular glycopyrrolate 0.2 mg and midazolam 2 mg. Upon arrival at the operating room, routine anesthesia monitoring including ECG, pulse oximetry, non-invasive arterial pressure, side-stream capnography were applied. Anesthesia was induced with alfentanil 10 µg/kg and propofol 2 mg/kg, intravenously. Rocuronium 0.8 mg/kg was injected intravenously to facilitate nasotracheal intubation. Reinforced tubes were used with 7.0 mm ID and 6.5 mm ID for men and women, respectively. The pressure of the tracheal cuff was maintained at 30 cm H2O throughout the procedure. After induction, dexamethasone 5 mg was administered intravenously. Anesthesia was maintained with desflurane 4-7 vol% in 50% N2O/O2 mixture. No additional opioid was administered during surgery. All patients received ketorolac 30 mg and metoclopromide 10 mg intravenously 30 min before the end of surgery. Neuromuscular blocking was reversed by pyridostigmine and glycopyrrolate 5 min before the end of surgery and then the administering gas concentration was gradually decreased. Alfentanil diluted in 10 ml normal saline was injected slowly for 30 s when the end-tidal vol% of desflurane was decreased to 3%. After surgery when the drapes were removed, the pharynx was suctioned gently, the vaporizer of desflurane was turned off and fresh gas flow was increased to 6 L/min in 100% oxygen. During the emergence phase, the patient was stimulated verbally or with gentle tactile stimulation on the shoulder every 20 s. From time zero, which was defined as a discontinuation of desflurane, the following variables were measured: time to the first response to the verbal command 'pen your eyes', time to tracheal extubation, end-tidal desflurane concentration at the first response and extubation, and the grade of coughing episodes (0: no cough; 1: mild, single cough; 2: moderate, more than 1 cough lasting <5 s; 3: severe, sustained for more than 5 s). These values were recorded by another observer blinded to the alfentanil dose. Extubation was performed when the patient could follow verbal commands and the tidal volume was sufficient. The mean arterial pressure (MAP) and heart rate (HR) were also recorded every 2 min from time 0 to 6 min after extubation. The presence of postoperative nausea and vomiting (PONV) and the modified Aldrete score [15] 30 min after admission to the post anesthesia care unit (PACU) were recorded.
Alfentanil dose was determined to be 16 µg/kg for the first patient, referring to a previous report [12]. Cough was defined as a sudden contraction of the abdomen. The success of suppressing cough during emergence was defined as grade 0 or 1 of any coughing episode. If cough suppression was successful, the alfentanil dose was decreased by 2 µg/kg in the consecutive patient. On the other hand, the dose was increased by 2 µg/kg in the consecutive patient if it failed.
Patient data was reported as the mean ± standard deviation (SD). Statistical analysis was performed using the SPSS package (SPSS 13.0 for windows, SPSS Inc, Chicago, IL). According to Dixon's up-and-down method [16], the study continued until seven pairs of successful-failed suppressing emergence cough had occurred. The 50% effective dose (ED50) of alfentanil for suppressing cough during emergence was defined as the mean of the median cross-over dose. The data was also subjected to isotonic regression estimators to calculate the 95% effective dose (ED95) and the 95% confidence interval (CI). An adjusted response probability was easily calculated by the pooled adjacent-violators algorithm (PAVA) and the CI was estimated using a bootstrapping approach [17]. The hemodynamic changes during emergence were analyzed by repeated measures ANOVA. A P value < 0.05 was considered significant.
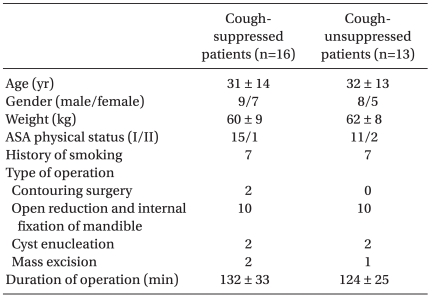
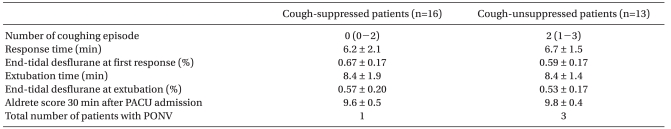
Twenty-nine patients were enrolled in this study when seven pairs of successful-failed suppressing emergence cough had occurred (Fig. 1). According to the success or failure in suppressing emergence cough, they were divided into a cough-suppressed group and a cough-unsuppressed group. Patient demographic data and operative characteristics in cough-suppressed group and cough-unsuppressed group were similar (Table 1). During the emergence phase, there were no significant differences in the response time, extubation time, and end tidal desflurane concentration at the response or extubation between the two groups. The two groups were also comparable with respect to the number of patients with PONV and the modified Aldrete score 30 min after admission to the PACU (Table 2). Hemodynamic changes during the emergence phase between the two groups were not significantly different. Within each group, the HR were similar compared to the baseline value but the MAP increased significantly after extubation compared to the baseline value (Fig. 2).
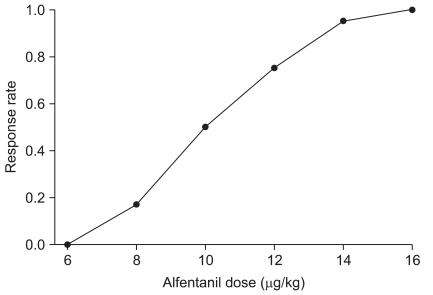
The ED50 of alfentanil for suppressing cough during emergence from desflurane anesthesia was 9.3 ± 1.5 µg/kg according to Dixon's up-and-down method (Fig. 1). Isotonic regression, estimated from the Pooled-adjacent-violators algorithm (PAVA) response rate, revealed that an ED50 and ED95 of alfentanil were 10.0 µg/kg (95% CI 6.8-13.2 µg/kg) and 14.0 µg/kg (95% CI 7.7-19.4 µg/kg), respectively (Fig. 3).
This is the first study on the dose-response of alfentanil for suppressing emergence cough. In this study, the ED95 of alfentanil for suppressing cough during emergence from desflurane anesthesia was 14.0 µg/kg.
Most upper airway reflex responses including the cough reflex have been observed in humans anesthetized lightly with inhalational anesthetics [18,19]. During emergence from inhalational anesthesia, stimulation of the airway mucosa in the state of light anesthesia can cause cough responses, interfering with smooth emergence. Among these airway reflex responses, the cough reflex appears to be most vulnerable to the depressant effect of fentanyl [20]. Opioid agonists, such as fentanyl, are centrally acting antitussive agents [21]. Recent studies reported that the antitussive effects of opioids are mediated predominantly by mu-and kappa-opioid receptors in the central nervous system [22,23].
Several studies reported that short-acting opioids, such as alfentanil and remifentanil, suppressed emergence cough induced by the endotracheal tube. The continuous infusion of low-dose remifentanil during emergence from isoflurane anesthesia reduced the incidence and severity of emergence cough [13]. Lee et al. [14] found that the EC95 of the effect site concentration of remifentanil to prevent coughing at emergence from propofol-remifentanil anesthesia was 2.14 ng/ml. However, a bolus of 1 µg/kg remifentanil at the end of surgery did not reduce the incidence of cough during emergence from isoflurane anesthesia [24]. On the other hand, a single bolus injection of alfentanil (15 µg/kg) during emergence decreased the incidence of emergence cough from 82% to 6% [12]. For this reason, alfentanil may be more suitable for single bolus administration to decrease emergence cough. Alfentanil bolus administration is a simple method compared to remifentanil continuous infusion. However, no dose-finding study on the clinical effective dose of alfentanil to suppress emergence cough have been conducted. Therefore, the clinically effective single dose of alfentanil for suppressing emergence cough was determined using Dixon's up-and-down method in this study.
A single bolus administration of alfentanil at the end of surgery did not prolong the extubation time compared to the control group [12]. Similarly, the extubation time in this study after the administration of alfentanil was <10 min in both groups, which is a clinically acceptable emergence time. In the hemodynamic changes during emergence, HR was relatively stable but the MAP increased significantly after extubation in both groups. These results were on various doses of alfentanil. Therefore, more studies of the recovery profile or hemodynamic changes on this optimal alfentanil dose are required.
These results have some limitations when applied to clinical practice. The desflurane used in this study has a direct effect on pungency and airway irritability [25]. This property of desflurane can affect the effective clinical dose of alfentanil for suppressing cough during emergence. The optimal dose of alfentanil may be different in preventing emergence cough for other anesthetics, such as sevoflurane or propofol. In addition, the criterion of success in suppressing emergence cough was not absence of cough. Therefore, the alfentanil dose proposed in this study may not be sufficient for situations requiring absolute smooth emergence.
In conclusion, the 95% effective dose (ED95) of alfentanil for suppressing cough during emergence from desflurane anesthesia is 14 µg/kg using Dixon's up-and-down method and isotonic regression. A single bolus administration of alfentanil during emergence from desflurane anesthesia is useful for suppressing emergence cough.
References
1. Fagan C, Frizelle HP, Laffey J, Hannon V, Carey M. The effects of intracuff lidocaine on endotracheal tube induced emergence phenomena after general anesthesia. Anesth Analg. 2000; 91:201–205. PMID: 10866913.
2. Gonzalez RM, Bjerke RJ, Drobycki T, Stapelfeldt WH, Green JM, Janowitz MJ, et al. Prevention of endotracheal tube-induced coughing during emergence from general anesthesia. Anesth Analg. 1994; 79:792–795. PMID: 7943794.

3. Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998; 87:1170–1174. PMID: 9806703.

4. Leech P, Barker J, Fitch W. Proceedings: changes in intracranial pressure and systemic arterial pressure during the termination of anaesthesia. Br J Anaesth. 1974; 46:315–316. PMID: 4451611.
5. Holden R, Morsman CD, Butler J, Clark GS, Hughes DS, Bacon PJ. Intra-ocular pressure changes using the laryngeal mask airway and tracheal tube. Anaesthesia. 1991; 46:922–924. PMID: 1750590.

6. Bidwai AV, Bidwai VA, Rogers CR, Stanley TH. Blood-pressure and pulse-rate responses to endotracheal extubation with and without prior injection of lidocaine. Anesthesiology. 1979; 51:171–173. PMID: 453622.

7. Neelakanta G, Miller J. Minimum alveolar concentration of isoflurane for tracheal extubation in deeply anesthetized children. Anesthesiology. 1994; 80:811–813. PMID: 8024135.

8. Koga K, Asai T, Vaughan RS, Latto IP. Respiratory complications associated with tracheal extubation. Timing of tracheal extubation and use of the laryngeal mask during emergence from anaesthesia. Anaesthesia. 1998; 53:540–544. PMID: 9709138.
9. Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anaesthesia. Anesth Analg. 2004; 99:1253–1257. PMID: 15385385.
10. Fagan C, Frizelle HP, Laffey J, Hannon V, Carey M. The effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anaesthesia. Anesth Analg. 2000; 91:201–205. PMID: 10866913.
11. Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005; 49:1088–1091. PMID: 16095449.

12. Mendel P, Fredman B, White PF. Alfentanil suppresses coughing and agitation during emergence from isoflurane anesthesia. J Clin Anesth. 1995; 7:114–118. PMID: 7598918.

13. Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009; 108:1157–1160. PMID: 19299779.

14. Lee B, Lee JR, Na S. Targeting smooth emergence: the effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br J Anaesth. 2009; 102:775–778. PMID: 19411668.

15. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995; 7:89–91. PMID: 7772368.

16. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991; 15:47–50. PMID: 2052197.

17. Pace NL, Stylianou MP. Advances in and limitation of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007; 107:144–152. PMID: 17585226.
18. Nishino T, Hiraga K, Mizuguchi T, Honda Y. Respiratory reflex responses to stimulation of tracheal mucosa in enflurane-anesthetized humans. J Appl Physiol. 1988; 65:1069–1074. PMID: 3182475.

19. Nishino T, Kochi T, Ishii M. Differences in respiratory reflex responses from the larynx, trachea, and bronchi in anesthetized female subjects. Anesthesiology. 1996; 84:70–74. PMID: 8572356.

20. Tagaito Y, Isono S, Nishino T. Upper airway reflexes during a combination of propofol and fentanyl anesthesia. Anesthesiology. 1998; 88:1459–1466. PMID: 9637637.

21. Miller RD. Anesthesia. 1994. 4th ed. New York: Churchill Livingstone;p. 291–388.
22. Kamei J, Tanihara H, Kasuya Y. Antitussive effects of two specific kappa-opioid agonists, U-50, 488H and U-62, 066E, in rats. Eur J Pharmacol. 1990; 187:281–286. PMID: 2272363.
23. Kamei J, Tanihara H, Kasuya Y. Modulation of mu- mediated antitussive activity in rats by a delta agonist. Eur J Pharmacol. 1991; 203:153–156. PMID: 1665789.
24. Shajar MA, Thompson JP, Hall AP, Leslie NA, Fox AJ. Effect of a remifentanil bolus dose on the cardiovascular response to emergence from anaesthesia and tracheal extubation. Br J Anaesth. 1999; 83:654–656. PMID: 10673886.

25. Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC. Clinical anesthesia. 2009. 6th ed. Philadelphia: Lippincott Williams&Wilkins;p. 434.
Fig. 1
Success or failure of suppressing coughing during emergence from desflurane anaesthesia on the determined alfentanil dose. The mean ± SD of the median alfentanil dose of seven success-failure pairs is 9.3 ± 1.5 µg/kg.
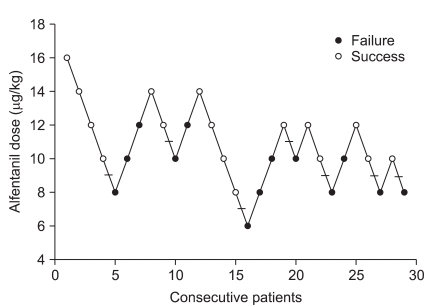
Fig. 2
Hemodynamic changes during the emergence phase. Time 0: at discontinuation of desflurane, time 1: 4 min before extubation, time 2: 2 min before extubation, time 3: at extubation, time 4: 2 min after extubation, time 5: 4 min after extubation, time 6: 6 min after extubation. There was no significant differences between the two groups. *P < 0.05 compared to the baseline.
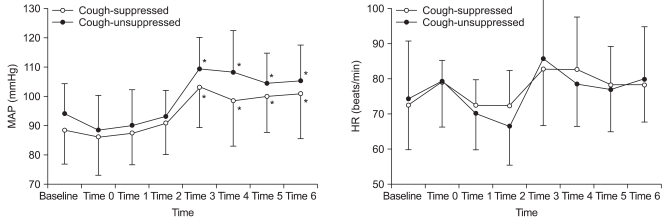
Fig. 3
Pooled-adjacent-violators algorithm (PAVA) response rate. The ED50 and ED95 (95% confidence interval), which were estimated from the PAVA response rate of the alfentanil dose for suppressing emergence cough from desflurane anesthesia, were 10.0 µg/kg (6.8-13.2 µg/kg) and 14.0 µg/kg (7.7-19.4 µg/kg), respectively.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download