This article has been
cited by other articles in ScienceCentral.
Abstract
Background
General anesthesia often produces some degree of hypothermia and hypothermia causes much more blood loss during surgery than normothermia. Electrically heated humidifiers (EHHs) have been used for patients under general anesthesia and in the intensive care unit. However, the benefits of the EHH have not been widely reported in the literature.
Methods
Patients scheduled for posterior lumbar spine fusion, were randomly assigned to a mechanically ventilated with EHH circuit group or to a conventional respiratory circuit group. Their tympanic membrane temperature was monitored every 30 min after induction up to 180 min, and perioperative blood losses, transfusion requirements during surgery, and other complications were noted.
Results
Patients in the control group (n = 40) showed a lower mean body temperature at all times than immediately after induction, while the EHH group (n = 40) showed a lower body temperature from 60 minute after induction comparing to the initial temperature. Furthermore, patients in the EHH group had a higher mean body temperature than patients in the control group during surgery (35.9 ± 0.4 vs 35.4 ± 0.5, P < 0.001). Mean intraoperative blood loss (9.75 ± 5.4 vs 7.48 ± 3.9, P = 0.035) and transfusion requirements (57.5% vs 25%, P = 0.006) were significantly less in the EHH group, but postoperative blood loss, duration of hospitalization, and other complications were not significantly different in the two study groups.
Conclusions
The use of an electrically heated humidifier did not prevent a body temperature drop under general anesthesia. However, it helped maintain body temperature and was associated less blood loss and transfusion requirement during surgery.
Go to :

Keywords: Blood loss, Complication, Electrically heated humidifier, Hypothermia, General anesthesia, Transfusion
Introduction
General anesthesia impairs thermal regulation and produces hypothermia in unwarmed surgical patients [
1]. Despite some disagreement, hypothermia appears to increase blood loss [
2], which impairs platelet function, primarily by impairing the release of thromboxane A2, which is necessarily for formation of an initial platelet plug and it also impairs the function of enzymes in the coagulation cascade [
3,
4]. Hypothermia has also been associated with wound infection [
5], shivering [
6], perioperative cardiac events [
7], and prolonged recovery and hospitalization [
8]. Therefore, many strategies have been devised to prevent intraoperative hypothermia, e.g., a forced-air warming system [
9], fluid warmers [
10], circulating-water mattresses [
11], and insulation of the skin surface [
12].
Electrically heated humidifiers (EHHs) add moisture and heat to inspiratory gases from temperature-regulated water reservoirs, and can prevent water loss from the tracheobronchial mucous membrane. Furthermore, EHH can theoretically maintain body temperature by reducing evaporation of water from the surface of mucous membranes and providing some convective energy to intubated patients [
13]. However, in practice, few studies have been carried out about the effects of EHH on the body temperature of patients receiving general anesthesia.
Therefore, in this trial, we evaluated the effects of EHH on the body temperature, blood loss and other complications of the patients receiving spinal surgery under general anesthesia.
Go to :

Materials and Methods
This was a single-center, prospective, randomized, single-blinded study, in which the effect of an electrically heated humidifier on body temperature was investigated in patients undergoing posterior lumbar spinal fusion surgery. The institutional review board of the hospital approved the protocol and written informed consent was obtained from all participants.
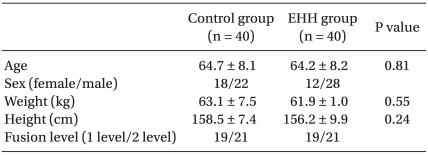
Patients, who were aged 25-75 years, had an American Society of Anesthesiologists physical status of I or II, and were scheduled to receive posterior lumbar spinal fusion with metal device instrumentation within two spinal levels (
Table 1). Patients with any of the following conditions were excluded; coagulation disorder, which we defined as a partial prothromboplastin time exceeding 38 s, a prothrombin time < 80% of normal, platelet count < 100,000/mm
3, consumption of aspirin within a week of surgery, uncontrolled hypertension and diabetes, body temperature > 37.0℃ on the day of surgery, or infection at the lumbar site.
Table 1

Patients were not premedicated on the day of surgery. Anesthesia was induced with propofol (Popol®, Hana Pharmacy, Seoul; 1 to 1.5 mg/kg IV), and remifentanil (Ultiva®, GSK, UK; 1 µg/kg) and tracheal intubation was facilitated with rocuronium (Esmeron®, Organon, Netherlands; 0.6 mg/kg). Anesthesia was maintained with 50% oxygen in air at a total flow of 3 L/min with sevoflurane (Abbott, UK), and remifentanil infusion, as well as-rocuronium as needed. Tidal volume was 8-10 ml/kg and respiratory rate was 8-12/min, and end tidal CO2 was maintained at 35-40 mmHg. A mean arterial pressure of 60 to 70 mmHg was achieved with sevoflurane (1.0 to 2.0% end expiratory concentration) and 0.1 to 0.5 µg/kg/min of remifentanil. ECG, non-invasive blood pressure (or determined by radial artery cannulation), oxygen saturation, and expired gas concentrations were monitored.
Patients were assigned, using a computer-generated random table, either to receive a respiratory circuit with an EHH (set at 37℃; MR 290 autofeed chamber, Fisher & Paykel Healthcare, Auckland, New Zealand) 15 min before induction (n = 40) or a conventional respiratory circuit (n = 40). A North American Dräger anesthetic machine (Cato, Dräger, Germany, Lübeck) was used with soda lime as a CO2 absorber in the anesthesia system. Tympanic membrane temperatures (OMRON, mc-505, Kyoto, Japan) were measured during the pre-induction period, immediately after induction and every 30 minutes up to 180 minutes after induction. After all patients had been placed in the prone position, the legs and hip area were covered with surgical drapes, the upper extremities, thoracic area, and head were covered with two-layers of blankets, and a forced air-warmer (Bair-Hugger) set 40-42℃ was applied between the blanket layers, as is usually done in our hospital. One surgeon who was not aware of our study performed the procedure. At the end of surgery, a residual neuromuscular blockade was antagonized with glycopyrrolate (Tabinul, Hana Pharmacy, Seoul; 0.2 mg IV) and pyridostigmine (Phygmin, Hana Pharmacy, Seoul; 10 mg IV). All patients were extubated and admitted in the recovery room. The ambient operating room temperature was set at 20℃. Fluids were administered at room temperature except blood, which was warmed to 36℃. Blood loss was calculated from observed quantities in suction chambers, minus irrigation fluids, plus increase in weight of sponges and cottonoid patties. Packed RBCs were transfused if more than 30% of blood volume was lost, or hematocrit at introperative blood gas analysis was less than 27%. An anesthesiologist who was not aware of our study visited patients at 24 and 48 hours postoperatively and recorded the quantities of blood in a hemovac (REF 2505, Zimmer, USA). We investigated the presence of postoperative complication (wound infection, respiratory problems, myocardiac infarction) and the length of hospitalization retrospectively.
The size of the study population used for this trial was 80 patients. Based on a review [
9], the lowest temperature of the control group was 34.8 ± 0.6℃, and we considered a 0.4℃ increase in the EHH group [
13]. On the basis of the formula for normal theory, this study needed 34 patients in each group for a type I error (α) of 0.05 and a power (1-β) of 0.80 to detect a 0.4℃ increase in the EHH group.
Demographic data and infused fluid volume are presented as mean and SDs and group values were compared by the Student's t test. Categorical data for each group were compared using a chi-square test or Fisher's exact test as appropriate. A repeated measures ANOVA with post hoc Tukey correction was used to compare group body temperatures at specific times. SPSS (Chicago, IL, USA) 14.0 was used for all analyses. A P < 0.05 was considered statistically significant.
Go to :

Results
There were no drop-outs from either group during surgery or hospital stays, and no patients showed surgical complications (wound infection, perioperative myocardial infarction, resurgery, or respiratory problems).
Changes in mean tympanic membrane temperature in the two groups during surgery are shown in
Table 2. When body temperature was measured with repeated measures ANOVA with respect to time, a significant difference was observed between the two groups (P < 0.001). In particular, the EHH group had a significantly higher mean tympanic membrane temperature than the control group during surgery. In the control group, all measured temperatures during surgery were significantly lower than immediately after induction. In the EHH group, the mean temperature was also significantly lower than immediately after induction except 30 minutes after induction. The lowest temperature of the EHH group was 35.9 ± 0.4℃ at 180 min after induction and in the control group, 35.4 ± 0.5℃ at the same time.
Table 2


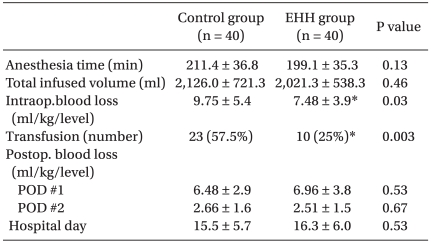
Table 3 depicts clinical outcomes in the two groups. The mean blood loss during surgery was significantly lower in the EHH group than in the control group (7.48 ± 3.9 ml/kg/level versus 9.75 ± 5.4 ml/kg/level, P = 0.035), and the rate of patients who required transfusion was greater in the control group (57.5% versus 25%, P = 0.003, RR=2.3, 95% CI 1.26-4.19) during the intraopeative period. However, the mean postoperative blood losses and mean period of hospitalization did not differ between the two groups.
Table 3

Go to :

Discussion
According to the study performed by Déry et al. [
14], during nasal respiration, inspired air is warmed and humidified by the evaporation of water from the surfaces of mucous membranes, and this evaporative process induces a loss of energy that results in mucous membrane cooling. During nasal respiration of ambient air at a rate of 7 L per minute, total water loss due to humidification in the nasal and lower respiratory tract of approximately 24 mg/L occurs. Because the quantity of energy that is required to evaporate 1 g of water at body temperature is 0.585 kcal, the water loss of 24 mg/L corresponds to an energy loss of 0.014 kcal/L. Consequently, in a typical minute ventilation of 7 L, an adult human being loses 250 ml/d. This corresponds to an energy loss of approximately 140 kcal/d or about 6 kcal/h. Furthermore, warming inspired air from 22℃ to 37℃ requires an energy input of 1.2 kcal/h. Thus, total respiratory energy loss is 10 to 12% of the total heat produced by a person at rest (60-100 kcal/h). Breathing dry air increases these energy losses because it induces more evaporation in the respiratory tract.
During intubation, the nasopharynx is bypassed functionally. Therefore, the conditioning of the respiratory air is restricted to the limited heat and moisture retaining capacities of the tracheal tube and Y-piece, and thus, respiratory air is humidified in regions not normally exposed to dry air. This situation is exacerbated by the fact that medical respiratory gases contain substantially less moisture than dose ambient air. Therefore, the quantities of water and energy that are required to condition the air to alveolar conditions is greater than under physiologic circumstances. Accordingly, the artificial humidification of respiratory air is necessary when the upper airway is bypassed with a tracheal tube.
Electrically heated humidifiers add moisture and heat to inspired air using temperature-regulated water reservoirs. Furthermore, EHHs can increase the water content of 7 mg/L more than heat and moisture exchangers (HMEs), which corresponds to an energy equivalent of 4.5 kcal/h more than with HMEs [
13].
The first rapid decrease in body temperature observed during general anesthesia is related to the internal distribution of heat from the central compartment to the peripheral compartments, which is due to the peripheral vasodilatation by anesthetic drugs [
15]. The second phase of the hypothermic response reflects body heat loss to the environment as a result of physical processes (radiation, evaporation, convection, and conduction). The greatest pathway of heat loss is radiation loss, which accounts for as much as 50% of the total heat loss [
16]. Heat loss of this route is related to the difference in temperature between the patient and surrounding environment. Convection is the second most important heat loss pathway and it is due to heat losses from the skin, open cavities, and respiratory tract [
17].
Goldberg et al. [
18] found that an EHH could not prevent a drop in temperature in major operations compared to a control group. However, they did not conduct a sample size calculation, and recruited only a small number of patients. On the other hand, Stone et al. [
15] reported that an EHH can maintain body temperature better than no device and that an EHH effectively warmed patients with a low temperature. Park et al. [
19] reported that as compared with a control group, their EHH group showed a higher esophageal temperature from 45 to 180 minutes after induction and found that EHH could prevent the abrupt decrease of body temperature in the first 1 to 2 hours after anesthesia. However, these studies involved different anesthetic techniques and surgeries. Their patients underwent orthopedic, general and gynecological surgery. However, we administered similar anesthesia to all patients and studied patients underwent the same type of surgery.
Our results indicated that EHH could prevent the temperature drop that occurs 30 minute after induction, but cannot prevent the subsequent temperature drop. This implies that the EHH does not completely compensate during the first and second phasees of hypothermia. We believe that EHH prevented the first phase of hypothermia by reducing evaporation in the tracheobroncheal tree and reducing some heat convection. However, respiratory heat loss is smaller than radiative heat loss, and therefore, EHH cannot prevent the temperature drop in the second phase of hypothermia.
In the systemic review of the effects of hypothermia on blood loss and transfusion requirements, even mild hypothermia (< 2.0℃) was found to increases blood loss by an estimated 16% (CI 4-26%), and, mild hypothermia was found to significantly increase the relative overall risks for transfusion by approximately 22% (CI 3-37%) [
20]. Furthermore, in that previous study, the median of the mean body temperature was 35.6℃. Our results showed that the EHH group showed significantly less blood loss and transfusion requirements than the control group by amount of 32.5% (57.5% versus 25%). Recently, transfusion seems to be more harmful than previously believed [
21,
22]. However, in our study, the two groups' mean postoperative blood losses, which were determined using Hemovac quantities at 24 and 48 hours after surgery, were not different This fact suggests that the effect of hypothermia on the bleeding is temporary and reversible. In addition, no patients in either group experienced complications, such as perioperative myocardial problems, wound infections, or prolonged recovery or hospitalization.
Actually, spinal surgery presents difficulties in terms of warming because patients are placed in the prone position and only the legs can be covered with active warming devices. Furthermore, it is difficult to apply a forced-air warmer because of the surgical frame. Murat et al. [
9] reported that when a forced-air warmer was applied to the lower legs of children receiving spinal surgery, the mean body temperature was higher than in a control group. However, the time-dependent effects of active air-warming were not compared with the baseline temperature. In addition, the present study shows that skin insulation and passive air warming is not sufficient in this type of surgery. Other studies had also indicated that passive insulation decreased heat loss by only 30-50% and that it was unable to compensate for large intraoperative cutaneous and evaporative heat losses [
12,
23].
Some limitations to our study require consideration. First, we did not measure the body temperature in the recovery room or the proportion of the patients who shivered and felt a cold sensation. Second, we did not calculate health care utilization costs, although we did investigate durations of hospitalization, which was no different in the two groups. However, EHH units are high-cost equipment and thus, the balance between medical benefits and patient satisfaction on the one hand and cost savings on the other requires further study.
Summarizing, in the present study, an electrically heated humidifier (EHH) did not completely prevent a temperature drop during spinal surgery, but patients in the EHH group had a higher body temperature than patients in the control group. In addition, the EHH group showed less intraoperative blood loss and transfusion requirements than the control group.
Go to :

Acknowledgements
This study was supported by NHIMC Ilsan hospital research fund.
Go to :

References
1. Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995; 82:662–673. PMID:
7879935.

2. Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996; 347:289–292. PMID:
8569362.

3. Valeri CR, Khabbaz K, Khuri SF, Marquardt C, Ragno G, Feingold H, et al. Effect of skin temperature on platelet function in patients undergoing extracorporeal bypass. J Thorac Cardiovasc Surg. 1992; 104:108–116. PMID:
1614195.
4. Michelson AD, MacGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994; 71:633–640. PMID:
7522354.

5. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med. 1996; 334:1209–1215. PMID:
8606715.
6. Just B, Delva E, Camus Y, Lienhart A. Oxygen uptake during recovery following naloxone. Relationship with intraoperative heat loss. Anesthesiology. 1992; 76:60–64. PMID:
1729937.

7. Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Oslon KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997; 277:1127–1134. PMID:
9087467.

8. Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997; 87:1318–1323. PMID:
9416715.

9. Murat I, Bernière J, Conatant I. Evaluation of the efficacy of a forced-air warmer (Bair Hugger) during spinal surgery in children. J Clin Anesth. 1994; 6:425–429. PMID:
7986517.

10. Jeong SM, Hahm KD, Jeong YB, Yang HS, Choi IC. Warming of intravenous fluids prevents hypothermia during off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2008; 22:67–70. PMID:
18249333.

11. Ihn CH, Joo JD, Chung HS, Choi JW, Kim DW, Jeon YS, et al. Comparison of three warming devices for the prevention of core hypothermia and post-anaesthesia shivering. J Int Med Res. 2008; 36:923–931. PMID:
18831885.

12. Sessler DI, Schroeder M. Heat loss in human covered with cotton hospital blankets. Anesth Analg. 1993; 77:73–77. PMID:
8317751.
13. Rathgeber J. Devices used to humidify respired gases. Respir Care Clin N Am. 2006; 12:165–182. PMID:
16828689.
14. Déry R, Pelletier J, Jacques A, Clavet M, Houde JJ. Humidity in anaesthesiology. 3. Heated and moisture patterns in the respiratory tract during anaesthesia with the semi-closed system. Can Anaesth Soc J. 1967; 14:287–298. PMID:
6067948.
15. Stone DR, Downs JB, Paul WL, Perkins HM. Adult body temperature and heated humidification of anesthetic gases during general anesthesia. Anesth Analg. 1981; 60:736–741. PMID:
7197477.

16. Howell WH. Physiology and Biophysics. 1979. 20th ed. Philadelphia: Saunders;p. 105–112.
17. Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008; 109:318–338. PMID:
18648241.

18. Goldberg ME, Epstein R, Rosenblum F, Larijani GE, Marr A, Lessin J, et al. Do heated humidifiers and heat and moisture exchangers prevent temperature drop during lower abdominal surgery? J Clin Anesth. 1992; 4:16–20. PMID:
1540363.

19. Park HG, Im JS, Park JS, Joe JK, Lee S, Yon JH, et al. A comparative evaluation of humidifier with heated wire breathing circuit under general anesthesia. Korean J Anesthesiol. 2009; 57:32–37.

20. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008; 108:71–77. PMID:
18156884.

21. Koch CG, Khandwala F, Li L, Estafanous FG, Loop FD, Blackstone EH. Persistent effect of red cell transfusion on health-related quality of life after cardiac surgery. Ann Thorac Surg. 2006; 82:13–20. PMID:
16798179.

22. Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006; 34:1608–1616. PMID:
16607235.

23. Sessler DI, McGuire J, Sessler AM. Perioperative thermal insulation. Anesthesiology. 1991; 74:875–879. PMID:
2021204.

Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download