Abstract
Elevated peak inspiratory airway pressure (PIP) can occur during general anesthesia and is usually easily rectified. In rare circumstances it can lead to potentially fatal conditions such as tension pneumothorax. We report on a 77-year-old male patient admitted for a cervical laminoplasty. The preoperative chest radiograph showed normal findings and there was no medical history of allergy or underlying airway inflammation. Anesthesia induction and maintenance progressed uneventfully. However, 5 minutes after prophylactic antibiotic administration, PIP suddenly increased and blood pressure dropped. The operation was abandoned and the patient was moved to a supine position to perform chest radiography. Cardiac arrest occurred, and cardiopulmonary resuscitation was performed. The radiograph showed bilateral tension pneumothorax. Needle aspiration was immediately performed, and chest tubes were inserted. Ventilation rapidly improved and the vital signs normalized. The patient was discharged without sequelae on postoperative day 36.
An increase in the peak inspiratory airway pressure (PIP) during general anesthesia most often occurs when patients have underlying airway inflammation associated with conditions such as bronchial asthma, chronic obstructive pulmonary disease, or upper respiratory infection. Other causes of increased PIP include stimuli from laryngoscopy, improper positioning of an endotracheal tube, an endotracheal foreign body, and anaphylaxis from medication. Increased PIP often occurs during the induction and maintenance of anesthesia. The most common cause of increased PIP during anesthesia is anaphylaxis following administration of muscle relaxants or antibiotics, which accounts for up to 34% of cases [1]. Elevated PIP can usually be easily rectified using appropriate management. However, in rare cases elevated PIP can lead to serious ventilation impairment or pulmonary barotraumas, and this can result in potentially fatal complications such as tension pneumothorax [2].
A 77-year-old male presented to the department of neurosurgery with prolonged numbness and weakness in the limbs. Investigation revealed compression of the spinal cord by a herniated pulposus, and a cervical laminoplasty from the 3rd to 6th cervical vertebra was scheduled. The medical history included hypertension, diabetes, and a coronary artery bypass graft due to acute myocardial infarction 8 years prior. There was no history of allergy.
The patient was 158 cm tall, and weighed 60 kg. Preoperative laboratory tests and chest radiography returned normal findings (Fig. 1A). Echocardiography showed hypokinesia of the basal inferior wall, but an ejection fraction of 63%. Computed tomography of the coronary arteries showed that the graft vessels were satisfactorily patent. At the preanesthetic visit there were no complaints of dyspnea or chest discomfort.
Premedication was not used. Theatre monitoring included noninvasive blood pressure measurement, electrocardiography, pulse oximetry, and end-tidal carbon dioxide concentration (ETCO2) measurement. The vital signs before anesthesia were: a blood pressure (BP) of 161/78 mmHg, regular sinus rhythm with a heart rate (HR) of 50 beat/min and a peripheral oxygen saturation (SpO2) of 99%. Anesthesia was induced using lidocaine 40 mg, propofol 120 mg, and vecuronium 10 mg. After conducting mask ventilation for 3 minutes with O2 4 L/min, N2O 4 L/min, and sevoflurane 6 vol%, the trachea was intubated with an 8.0-mm armored endotracheal tube. Anesthesia was maintained using O2 1 L/min and N2O 1 L/min, and sevoflurane 1.5-2.5 vol%. The vital signs remained stable after intubation. Mechanical ventilation was performed with minute ventilation of 5.5 L/min, ETCO2 of 28-30 mmHg, and a PIP of 18 cmH2O. Subsequently, vecuronium 4 mg/hr was infused for continuous muscle relaxation. The patient was shifted to the prone position for the operation, and the PIP was 20 cmH2O at that stage. Twenty minutes after anesthesia induction, cefuroxime sodium 1.5 g was administered as a prophylactic antibiotic without any previous allergic tests. Five minutes later, the PIP suddenly increased to 33 cmH2O, ETCO2 slightly increased to 35 mmHg, and SpO2 dropped to 94% and then recovered and was maintained at 97-100%. The endotracheal tube was suctioned, but there was no evidence of a large amount of secretion. Ventilation impairment worsened, and PIP increased to 40 cmH2O, BP dropped to 66/28 mmHg and HR to 35 beats/min. We immediately converted to ambu bag manual ventilation, however severe resistance was experienced. Auscultation was difficult due to the surgical position and the operation field. Best possible use of a esophageal stethoscope revealed low breathing sounds. N2O administration was immediately ceased, and 100% O2 6 L/min was administered. Suspecting that an anaphylactic reaction had occurred, hydrocortisone 100 mg and vecuronium 3 mg were injected for suspected bronchial spasm. However, ventilation did not improve. Atropine 0.5 mg and epinephrine 10 µg were injected, and a dopamine 10 µg/kg/min infusion was commenced to maintain BP. However, BP decreased to 56/22 mmHg and HR to 30 beat/min. Arterial blood gas analysis (ABGA) showed a pH of 7.03, PaCO2 of 88 mmHg, PaO2 of 345 mmHg, and SaO2 of 100% (Table 1). Epinephrine 30 µg was administered under continuous infusion at 0.05 µg/kg/min. Chest radiography was then considered essential to determine whether there was a mechanical problem or pulmonary complication. However, this was difficult due to the iron frame of the operating table. Moreover, the patient was undergoing cervical spine surgery in a fixed prone position, and if cardiac arrest eventuated, it would have been very difficult to perform cardiopulmonary resuscitation (CPR). Therefore, the surgery was abandoned and the surgical wound promptly closed. Ventilation did not improve and breathing was scarcely heard during wound closure. The patient was placed in the supine position and chest radiography performed. Cardiac arrest occurred. Atropine 1 mg and epinephrine 1 mg were immediately injected twice while chest compression was conducted for 10 minutes. Subsequently, BP increased to 110/43 mmHg and HR to 48 beat/min. SpO2 decreased to 70% and ABGA showed a pH of 6.97, PaCO2 of 115 mmHg, and PaO2 of 30 mmHg. The chest radiograph revealed tension pneumothorax in both lungs (Fig. 1B). Needle aspiration was immediately performed, followed by the insertion of chest tubes into the thoracic cavity. Following tube insertion, ventilation improved and PIP decreased to 28 cmH2O. ABGA showed a pH of 7.32, PaCO2 of 51 mmHg, PaO2 of 399 mmHg, and SaO2 of 100%. The vital signs normalized, BP was 135/54 mmHg, and HR was 52 beat/min. The patient was transferred to the intensive care unit with intubation.
On the 2nd postoperative day (POD), extubation was performed without complication. On the 13th POD, follow-up chest radiography showed no abnormalities except atelectasis in both lungs (Fig. 1C). The chest tube was removed and the patient was discharged without sequelae on the 36th POD.
The present case is an example of allergic reaction-induced bronchial spasm followed by a sudden increase in PIP and ventilation impairment during maintenance of general anesthesia. This series of events caused tension pneumothorax which ultimately required CPR.
Elevated PIP is common during anesthesia and is generally corrected without serious complications. Westhorpe et al. [1] reported that PIP often increases by 56-75% during induction and maintenance of anesthesia, and Cheney et al. [3] reported increases of 25-43% during anesthesia maintenance. The causes of increased PIP during general anesthesia include stimulation from intubation, a large volume of bronchial secretion, sudden inhalation of highly concentrated anesthetic, underlying respiratory disease such as chronic bronchitis or asthma, and allergic reactions to anesthetics and antibiotics [4].
The most common clinical symptom of high airway pressure is hypercapnia, making ventilation of the lungs difficult and leading to considerable CO2 emission impairment from the alveoli. The second most common symptom is hypoxia. In the case of oxygen, this gas moves into the blood through the alveoli unilaterally from the outside. Therefore, if oxygen is forced under high pressure from the outside, it can move into the alveoli, maintaining the SaO2 level. However, when there are lesions causing an alveolar ventilation/perfusion mismatch or perfusion defects in the pulmonary capillaries due to a decrease in BP, hypoxia can occur [2].
In the present case, it appeared that complications developed following the administration of the prophylactic antibiotic, and PIP increased due to the increase in airway resistance. Furthermore, due to the ventilation impairment, PaCO2 increased and SpO2 temporarily decreased to 94% at the beginning of the PIP increase. After supplying 100% O2 at a high flow rate, SpO2 was maintained at 97-100%.
Pneumothorax can develop as a result of increased PIP [2]. The endotracheal pressure causing pneumothorax has yet to be identified, but it is known to be associated with increased barotrauma as the peak airway pressure increases [5]. The clinical signs and symptoms of pneumothorax are hypoxia and hypercapnia due to a decrease in lung volume caused by the sudden increase in intrathoracic pressure. When the volume of the pneumothorax is large enough to develop tension pneumothorax, as in our case, cardiac output decreases because venous circulation is blocked due to compression of the vena cava, atrium, and large veins by shifting of the mediastinum. This can eventually cause hypotension leading to circulatory collapse [6].
Lobera et al. [7] reported that anaphylaxis during anesthesia was due to antibiotics in 44% of cases and muscle relaxants in 37% of cases. In the present patient, although allergic test was not performed prior to administer cefuroxime sodium using in our case by the policy of our institution and neither did it after the event to confirm the allergic reaction considering his general condition, antibiotic-induced anaphylaxis appeared to be the initiating event. That anaphylaxis resulted in an increase in PIP, which in turn caused positive pressure ventilation which caused development of pneumothorax, and this eventually developed into tension pneumothorax.
Upon suspicion of a bronchial spasm, clinicians should increase the depth of anesthesia using anesthetic agents and muscle relaxants, and also administer a bronchodilator such as a β-adrenergic agent. Aminophylline can also be administered to improve ventilation [2]. Moreover, although the mechanism is not clear, an injection of hydrocortisone can be effective, but this takes hours to have a clinical effect [8]. MgSO4 is effective for patients who experience a bronchial spasm [9]. In patients with respiratory diseases, a lack of response to such treatments should result in immediate transfer to an intensive care unit to establish ventilation. If pneumothorax occurs due to complications associated with a bronchial spasm, N2O administration should be immediately ceased and 100% oxygen should be given. A large-bore needle should also be inserted into the 2nd intercostal space for aspiration of air from the thorax. Finally, water-sealed drainage via chest tubes should be instigated. In cases of cardiovascular collapse due to tension pneumothorax, BP should be maintained using vasoconstrictors and inotropics.
The present report describes a case of a sudden increase in PIP after antibiotic administration which then developed to the potentially fatal condition of tension pneumothorax. This occurred in a patient with no history of allergy, no underlying respiratory inflammation and no abnormal findings during anesthesia induction. The planned surgery, like this case in the fixed prone position, was abandoned in order to obtain chest radiographs and to prepare for life-saving CPR if cardiac arrest occurred, which did indeed eventuate. In the present patient, this course of action resulted in recovery with no obvious sequelae. This case highlights that potentially fatal tension pneumothorax can develop in patients who may be considered at low risk of such a complication, and that a timely decision to abandon planned surgery, especially prompt CPR is not available, may be crucial in avoiding further serious complications or death.
References
1. Westhorpe RN, Ludbrook GL, Helps SC. Crisis management during anaesthesia: bronchospasm. Qual Saf Health Care. 2005; 14:e7. PMID: 15933304.

2. Oh SC, Son YS, Nam SW, Yoon KJ. Bronchospasm during the Maintenance of General Anesthesia. Korean J Anesthesiol. 2005; 49:532–537.
3. Cheney FW, Posner KL, Caplan RA. Adverse respiratory events infrequently leading to malpractice suits. A closed claims analysis. Anesthesiology. 1991; 75:932–939. PMID: 1741513.

4. Kim SW, Kim JG, Lee JS, Kwon BY. Acute attack of asthma and pulmonary collapse after tracheal intubation. Korean J Anesthesiol. 1995; 28:722–727.
5. Haake R, Schlichtig R, Ulstad DR, Henschen RR. Barotrauma. Pathophysiology, risk factors, and prevention. Chest. 1987; 91:608–613. PMID: 3549176.
6. Moon JE, Ahn HJ, Kim JA. Bilateral tension pneumothorax and bronchospasm in the patient with recent history of croup: A case report. Korean J Anesthesiol. 2007; 52:724–727.
7. Lobera T, Audicana MT, Pozo MD, Blasco A, Fernández E, Cañada P, et al. Study of hypersensitivity reactions and anaphylaxis during anesthesia in Spain. J Investig Allergol Clin Immunol. 2008; 18:350–356.
8. Barnes PJ. Mechanisms of action of glucocorticoids in asthma. Am J Respir Crit Care Med. 1996; 154:S21–S26. discussion S6-7. PMID: 8756783.

9. Skobeloff EM, Spivey WH, McNamara RM, Greenspon L. Intravenous magnesium sulfate for the treatment of acute asthma in the emergency department. JAMA. 1989; 262:1210–1213. PMID: 2761061.

Fig. 1
Chest radiographs. (A) Preoperative chest radiograph. (B) Intraoperative chest radiograph. Note the bilateral tension pneumothorax. (C) Postoperative 13th day chest radiograph. Note the resolved pneumothorax.
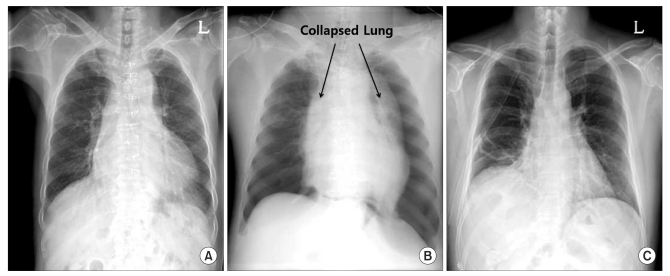
Table 1
Serial Changes in Arterial Blood Gas Analysis Data

pH: hydrogen ion concentration, PaCO2: partial pressure of arterial carbon dioxide, PaO2: partial pressure of arterial oxygen, BE: base excess, HCO3-: bicarbonate, SaO2: oxygen saturation, FiO2: fraction of inspired oxygen, UC: uncheckable, min: minute, hr: hour, ins.: insertion, ICU: intensive care unit, adm: administration, POD: postoperative day.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download