2. Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004; 350:1722–1730. PMID:
15102998.

3. Todres ID, Fugate JH. Critical Care for infants and children. 1996. Newyork: Little Brown & Co.
4. Goetting MG, Paradis NA. High-dose epinephrine improves outcome from pediatric arrest. Ann Emerg Med. 1991; 20:22–26. PMID:
1984722.
5. Bjørshol CA, Søreide E, Torsteinbø TH, Lexow K, Nilsen OB, Sunde K. Quality of chest compressions during 10 min of single-rescuer basic life support with different compression: ventilation ratios in a manikin model. Resuscitation. 2008; 77:95–100. PMID:
18207627.
6. Vento M, Saugstad OD. Resuscitation of the term and preterm infant. Semin Fetal Neonatal Med. 2010; 15:216–222. PMID:
20451481.

7. Dalens B. Regional anesthesia in pediatrics. Ann Fr Anesth Reanim. 1989; 8:51–66. PMID:
2653120.
8. Thomas JM, Schug SA. Recent advances in the pharmacokinetics of local anesthetics. Long-acting amide enantiomers and continuous infusion. Clin Pharmacokinet. 1999; 36:67–83. PMID:
9989343.
9. Karmakar MK, Kwok WH. Coté CJ, Lerman J, Todres D, editors. Ultrasound-Guided Regional Anesthesia. A Practice of Anesthesia for Infants and Children. 2009. 4th ed. Philadelphia: Saunders Elsevier;p. 911–938.

10. Flandin-Bléty C, Barrier G. Accidents following extradural anesthesia in children. The results of a retrospective study. Paediatr Anaesth. 1995; 5:41–46. PMID:
8521309.
11. Giaufre E, Dalens B, Gombert A. Epidemiology and morbidity of regional anesthesia in children: a one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg. 1996; 83:904–912. PMID:
8895261.
12. Tsui BC. Innovative approaches to neuraxial blockade in children: the introduction of epidural nerve root stimulation and ultrasound guidance for epidural catheter placement. Pain Res Manag. 2006; 11:173–180. PMID:
16960634.

13. Tsui BC, Suresh S. Ultrasound imaging for regional anesthesia in infants, children and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology. 2010; 112:719–728. PMID:
20179511.
14. Eger EI 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965; 26:756–763. PMID:
5844267.
15. Paul M, Fisher DM. Are estimates of MAC reliable? Anesthesiology. 2001; 95:1362–1370. PMID:
11748393.

16. Coté CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Coté CJ, Lerman J, Todres D, editors. Pharmacokinetics and Pharmakology of Drugs Used in Children. A Practice of Anesthesia for Infants and Children. 2009. 4th ed. Philadelphia: Saunders Elsevier;p. 100–119.
17. Maruyama K, Agata H, Ono K, Hiroki K, Fujihara T. Slow induction with sevoflurane was associated with complete atrioventricular block in a child with hypertension, renal dysfunction, and impaired cardiac conduction. Paediatr Anaesth. 1998; 8:73–78. PMID:
9483603.

18. Driessen JJ, van Oort AM, Booij LH. Severe myocardial ischaemia during mask induction of anaesthesia in an infant with unknown critical supravalvular aortic stenosis. Anaesthesia. 2003; 58:568–570. PMID:
12846623.

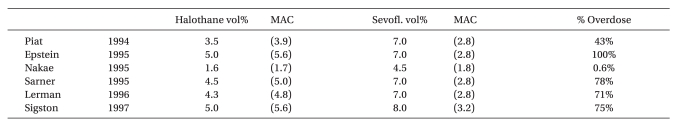
19. Piat V, Dubois MC, Johanet S, Murat I. Induction and recovery characteristics and hemodynamic responses to sevoflurane and halothane in children. Anesth Analg. 1994; 79:840–844. PMID:
7978397.

20. Epstein RH, Stein AL, Marr AT, Lessin JB. High concentration versus incremental induction of anesthesia with sevoflurane in children: a comparison of induction times, vital signs, and complications. J Clin Anesth. 1998; 10:41–45. PMID:
9526937.

21. Nakae Y, Miyabe M, Sonoda H, Kawana S, Namiki A. Comparison of intubating condition under sevoflurane and halothane anesthesia in pediatric patients. Masui. 1995; 44:239–243. PMID:
7739097.
22. Sarner JB, Levine M, Davis PJ, Lerman J, Cook DR, Motoyama EK. Clinical characteristics of sevoflurane in children: a comparison with halothane. Anesthesiology. 1995; 82:38–46. PMID:
7832332.
23. Lerman J, Davis PJ, Welborn LG, Orr RJ, Rabb M, Carpenter R, et al. Induction, recovery, and safety characteristics of sevoflurane in children undergoing ambulatory surgery: a comparison with halothane. Anesthesiology. 1996; 84:1332–1340. PMID:
8669674.
24. Sigston PE, Jenkins AM, Jackson EA, Sury MR, Mackersie AM, Hatch DJ. Rapid inhalation induction in children: 8% sevoflurane compared with 5% halothane. Br J Anaesth. 1997; 78:362–365. PMID:
9135351.

25. Holzki J, Kretz FJ. Changing aspects of sevoflurane in paediatric anaesthesia: 1975-99. Paediatr Anaesth. 1999; 9:283–286. PMID:
10411761.

26. Coté CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Coté CJ, Lerman J, Todres ID, editors. Pharmacokinetics and Pharmakology of Drugs Used in Children. A Practice of Anesthesia for Infants and Children. 2009. 4th ed. Philadelphia: Saunders Elsevier;p. 105.
27. Bhananker SM, Ramamoorthy C, Geiduschek JM, Posner KL, Domino KB, Haberkern CM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. 2007; 105:344–350. PMID:
17646488.

28. Paris ST, Cafferkey M, Tarling M, Hancock P, Yate PM, Flynn PJ. Comparison of sevoflurane and halothane for outpatient dental anaesthesia in children. Br J Anaesth. 1997; 79:280–284. PMID:
9389840.

29. Coté CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Coté CJ, Lerman J, Todres ID, editors. Pharmacokinetics and Pharmakology of Drugs Used in Children. A Practice of Anesthesia for Infants and Children. 2009. 4th ed. Philadelphia: Saunders Elsevier;p. 108.
30. Wodey E, Pladys P, Copin C, Lucas MM, Chaumont A, Carre P, et al. Comparative hemodynamic depression of sevoflurane versus halothane in infants: an echcardiographic study. Anesthesiology. 1997; 87:795–800. PMID:
9357880.
31. Haga S, Shima T, Momose K, Andoh K, Hashimoto Y. Anesthetic induction of children with high concentrations of sevoflurane. Masui. 1992; 41:1951–1955. PMID:
1479663.
32. Yli-Hankala A, Vakkuri A, Särkelä M, Lindgren L, Korttila K, Jäntti V. Epileptiform electroencephalogram during mask induction of anesthesia with sevoflurane. Anesthesiology. 1999; 91:1596–1603. PMID:
10598599.

33. Kaisti KK, Jääskeläinen SK, Rinne JO, Metsähonkala L, Scheinin H. Epileptiform discharges during 2 MAC sevoflurane anesthesia in two healthy volunteers. Anesthesiology. 1999; 91:1952–1955. PMID:
10598642.

34. Vakkuri A, Yli-Hankala A, Särkelä M, Lindgren L, Mennander S, Korttila K, et al. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiol Scand. 2001; 45:805–811. PMID:
11472278.

35. Jääskeläinen SK, Kaisti K, Suni L, Hinkka S, Scheinin H. Sevoflurane is epileptogenic in healthy subjects at surgical levels of anesthesia. Neurology. 2003; 61:1073–1078. PMID:
14581667.

36. Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth. 2005; 15:266–274. PMID:
15787916.

37. Särkelä MO, Ermes MJ, van Gils MJ, Yli-Hankala AM, Jäntti VH, Vakkuri AP. Quantification of epileptiform electroencephalographic activity during sevoflurane mask induction. Anesthesiology. 2007; 107:928–938. PMID:
18043061.

38. Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008; 107:1689–1703. PMID:
18931234.

39. Shichinohe Y, Masuda Y, Takahashi H, Kotaki M, Omote T, Shichinohe M, et al. A case of postoperative hepatic injury after sevoflurane anesthesia. Masui. 1992; 41:1802–1805. PMID:
1460759.
40. Iwanaga Y, Komatsu H, Yokono S, Ogli K. Serum glutathione S-transferase alpha as a measure of hepatocellular function following prolonged anaesthesia with sevoflurane and halothane in paediatric patients. Paediatr Anaesth. 2000; 10:395–398. PMID:
10886696.

41. Singhal S, Gray T, Guzman G, Verma A, Anand K. Sevoflurane hepatotoxicity: a case report of sevoflurane hepatic necrosis and review of the literature. Am J Ther. 2010; 17:219–222. PMID:
19455019.

42. Bösenberg AT, Murat I. Coté CJ, Lerman J, Todres D, editors. Pediatric anesthesia in developing countries. A Practice of Anesthesia for Infants and Children. 2009. 4th ed. Philadelphia: Saunders Elsevier;p. 1077–1084.
43. Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth. 2000; 10:419–424. PMID:
10886700.

44. Russell IA, Miller Hance WC, Gregory G, Balea MC, Cassorla L, DeSilva A, et al. The safety and efficacy of sevoflurane anesthesia in infants and children with congenital heart disease. Anesth Analg. 2001; 92:1152–1158. PMID:
11323338.

45. Cohen IT, Hannallah RS, Hummer KA. The incidenceof emergence agitation associated with deflurane anesthesia in children. Anesth Analg. 2001; 93:88–91. PMID:
11429345.
46. Lerman J, Hammer GB, Verghese S, Ehlers M, Khalil SN, Betts E, et al. Airway responses to desflurane during maintenance of anesthesia and recovery in children with laryngeal mask airways. Paediatr Anaesth. 2010; 20:495–505. PMID:
20456065.
47. Rosow C. Remifentanil: a unique opioid analgesic. Anesthesiology. 1993; 79:875–876. PMID:
7902031.
48. Akpek EA, Erkaya C, Donmez A, Mercan S, Esen A, Aslamaci S, et al. Remifentanil use in children undergoing congenital heart surgery for left-to-right shunt lesions. J Cardiothorac Vasc Anesth. 2005; 19:60–66. PMID:
15747271.

49. Ross AK, Davis PJ, Dear Gd GL, Ginsberg B, McGowan FX, Stiller RD, et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg. 2001; 93:1393–1401. PMID:
11726413.

50. Crawford MW, Hayes J, Tan JM. Dose-response of remifentanil for tracheal intubation in infants. Anesth Analg. 2005; 100:1599–1604. PMID:
15920180.

51. Choong K, AlFaleh K, Doucette J, Gray S, Rich B, Verhey L, et al. Remifentanil for endotracheal intubation in neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2010; 95:F80–F84. PMID:
20231228.

52. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998; 86:1307–1311. PMID:
9620525.

53. Blair JM, Hill DA, Wilson CM, Fee JP. Assessment of tracheal intubation in children after induction with propofol and different doses of remifentanil. Anaesthesia. 2004; 59:27–33. PMID:
14687095.

54. Nafiu OO, Kheterpal S, Morris M, Reynolds PI, Malviya S, Tremper KK. Incidence and risk factors for preincision hypotension in a noncardiac pediatric surgical population. Paediatr Anaesth. 2009; 19:232–239. PMID:
19143955.
55. Kanto J, Gepts E. Pharmacokinetic implications for the clinical use of propofol. Clin Pharmacokinet. 1989; 17:308–326. PMID:
2684471.

56. Holzki J, Aring C, Gillor A. Death after re-exposure to propofol in a 3-year-old child: a case report. Paediatr Anaesth. 2004; 14:265–270. PMID:
14996268.
57. Kill C, Leonhardt A, Wulf H. Lacticacidosis after short-term infusion of propofol for anaesthesia in a child with osteogenesis imperfect. Paediatr Anaesth. 2003; 13:823–826. PMID:
14617125.
58. Chukwuemeka A, Ko R, Ralph-Edwards A. Short-term low-dose propofol anaesthesia associated with severe metabolic acidosis. Anaesth Intensive Care. 2006; 34:651–655. PMID:
17061643.

59. Laquay N, Prieur S, Greff B, Meyer P, Orliaguet G. Propofol infusion syndrome. Ann Fr Anesth Reanim. 2010; 29:377–386. PMID:
20399595.
60. Ivani G, De Negri P, Conio A, Amati M, Roero S, Giannone S, et al. Ropivcacaine-clonidine combination for caudal blockade in children. Acta Anaesthesiol Scand. 2000; 44:446–449. PMID:
10757579.
61. Sillanpää M. Clonidine prophylaxis of childhood migraine and other vascular headache. a double blinded study of 57 children;. Headache. 1977; 17:28–31. PMID:
321395.
62. Tobias JD, Berkenbosch JW. Initial experience with dexmedetomidine in paediatric-aged patients. Paediatr Anaesth. 2002; 12:171–175. PMID:
11882231.

63. Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000; 59:263–268. PMID:
10730549.

64. Vilo S, Rautiainen P, Kaisti K, Aantaa R, Scheinin M, Manner T, et al. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br J Anaesth. 2008; 100:697–700. PMID:
18378546.

65. Potts AL, Larsson P, Eksborg S, Warman G, Lönnqvist PA, Anderson BJ. Clonidine disposition in children; a population analysis. Paediatr Anaesth. 2007; 17:924–933. PMID:
17767627.

66. Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006; 105:1098–1110. PMID:
17122572.

67. Tobin JR, Shafer SL, Davis PJ. Pediatric research and scholarship: another Gordian knot? Anesth Analg. 2006; 103:43–48. PMID:
16790623.

68. Fisher D. Do the right thing (or Do the market exclusivity thing?). Anesthesiology. 2006; 105:1074–1075. PMID:
17122566.

69. Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006; 104:667–674. PMID:
16571960.
70. Khine HH, Corddry DH, Kettrick RG, Martin TM, McCloskey JJ, Rose JB, et al. Comparion of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997; 86:627–631. PMID:
9066329.
71. Fisher DM. Highlight: comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997; 86V:27A.
72. Newth CJ, Rachman B, Patel N, Hammer J. The use of cuffed vs uncuffed endotracheal tubes in intensive care. J Pediatr. 2004; 144:333–337. PMID:
15001938.
73. Deakers TW, Reynolds G, Stretton M, Newth CJ. Cuffed endotracheal tubes in pediatric intensive care. J Pediatr. 1994; 125:57–62. PMID:
8021785.

74. Dubreuil C. Laryngotracheal stenosis in children. Pediatrie. 1987; 42:273–279. PMID:
3313266.
75. Wiel E, Vilette B, Darras JA, Scherpereel P, Leclerc F. Larngotracheal stenosis in children after intubation. Report of five cases. Paediatr Anaesth. 1997; 7:415–419. PMID:
9308067.
76. Ashtekar CS, Wardhaugh A. Do cuffed endotracheal tubes increase the risk of airway mucosal injury and post-extubation stridor in children? Arch Dis Child. 2005; 90:1198–1199. PMID:
16243883.

77. Holzki J, Laschat M, Puder C. Stridor is not a scientifically valid outcome measure for assessing airway injury. Paediatr Anaesth. 2009; 19(Suppl 1):180–197. PMID:
19572855.

78. Holzki J, Laschat M, Puder C. Iatrogenic damage to the pediatric airway. Mechanisms and scar development. Paediatr Anaesth. 2009; 19(Suppl 1):131–146. PMID:
19572852.

79. Weiss M, Dullenkopf A, Fischer JE, Keller C, Gerber AC. European Paediatric Endotracheal Intubation Study Group. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth. 2009; 103:867–873. PMID:
19887533.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download