Abstract
Background
The aim of this study was to investigate the effects of preoperative statin therapy on myocardial protection and morbidity endpoints following off-pump coronary bypass graft surgery (OPCAB) in patients with elevated serum high-sensitivity C-reactive protein (hs-CRP) levels.
Methods
Of the 492 patients who underwent multivessel OPCAB from March 2007 to February 2009, the records of 144 patients whose baseline hs-CRP level > 2 mg/L were reviewed. According to the history of preoperative statin therapy for at least one week, patients were classified as either statin group or control group (72 subjects each). Preoperative and operative characteristics and postoperative data including troponin (Tn)-T level and major morbidity endpoints were obtained and compared. Major morbidity endpoints were defined as permanent stroke, renal dysfunction, hemostatic re-exploration, deep sternal wound infection, and the number of patients requiring prolonged ventilation.
Results
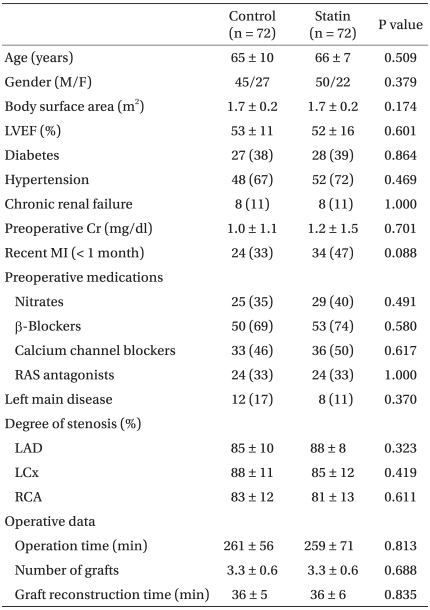
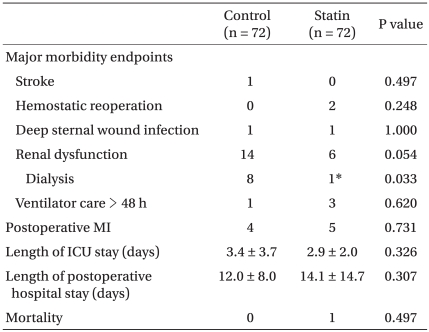
Preoperative and operative characteristics were similar between the two groups. There were no significant differences in the incidence of morbidity endpoints between the two groups, except for the number of patients requiring dialysis, which was significantly lower in the statin group (8 vs. 1, P = 0.033). Tn-T level at 24 h after surgery was also significantly lower in the statin group.
Go to : 
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor) exert multiple beneficial effects in terms of preventing cardiovascular events, including cardiac death, myocardial infarction (MI), stroke, and renal dysfunction, in patients undergoing percutaneous coronary intervention or with peripheral arterial disease [1-3]. These effects are attributable to both the lipid-lowering effect and pleiotropic effects beyond lipid lowering, such as anti-thrombotic and anti-inflammatory effects [4-6]. Consequently, statin therapy is commonly recommended for high-risk patients with normal average cholesterol levels as well as for those with hypercholesterolemia for the primary prevention of acute coronary events [7,8].
Despite the well-established beneficial role of statin therapy, conflicting results have been reported with regard to myocardial protection or the prevention of major morbidity endpoints following coronary artery bypass graft surgery (CABG) [9-15]. A recent JUPITER trial revealed beneficial effects of stains on reduction of major cardiovascular events in healthy patients with elevated high-sensitivity C-reactive protein (hs-CRP) levels [16]. The hs-CRP is a well-known marker of inflammatory status and is a reliable prognostic factor following CABG [17-19], and has already been used as an index of targeting statin therapy for prevention of coronary events [20,21]. Thus, it is reasonable to assume that patients with elevated hs-CRP would benefit most from statin therapy even in the surgical setting.
However, evidence is limited in terms of the effect of statin therapy on myocardial protection and morbidity endpoints in patients with elevated hs-CRP undergoing CABG. With regard to assessing the myocardial protective effect, multi-vessel off-pump CABG provides an optimal model for the presence of cumulative myocardial ischemia-reperfusion injury.
Therefore, the aim of the present study was to investigate the effects of preoperative statin therapy on myocardial protection and morbidity endpoints following off-pump CABG in patients with elevated hs-CRP.
Go to : 
A total of 492 electronic medical charts of patients who underwent elective isolated multi-vessel off-pump CABG from March 2007 to February 2009 were retrospectively reviewed. After excluding patients who were enrolled in other clinical studies and selecting 178 patients with baseline hs-CRP ≥ 2 mg/L according to the JUPITER trial [16], further selection was done for patients who received any kind of statin therapy for at least one week (statin group) or no statin therapy at all (control group). This retrieved a total of 144 patients, with 72 patients in each group. Of the 72 patients in the statin group, 25 received atorvastatin (10-30 mg/d), 18 received rosuvastatin (10-40 mg/d), 16 received simvastatin (10-40 mg/d), and 13 received pravastatin (10-40 mg/d).
All patients received standardized anesthetic and surgical care according to institutional guidelines as follows. They received 0.05-0.1 mg/kg of morphine intramuscularly as premedication 1 h before operation. Upon arrival at the operating room, standard monitoring devices were applied, including a pulmonary artery catheter (PAC, Swan-Ganz CCOmbo CCO/SvO2™, Edwards Lifesciences LLC, Irvine, CA, USA), which was inserted via the right internal jugular vein and connected to an analysis system (Vigilance™, Edwards Lifesciences LLC, Irvine, CA, USA) for continuous monitoring of cardiac index (CI) and mixed venous oxygen saturation (SvO2). Anesthesia was induced with intravenous midazolam (0.03-0.07 mg/kg) and sufentanil (1.5-2.0 µg/kg), and maintained with sevoflurane (0.8-1.5%) and continuous infusion of sufentanil (0.5-1.5 µg/kg/h). Neuromuscular blockade was achieved by administering rocuronium (0.9 mg/kg) and maintained with continuous infusion of vecuronium (1-2 µg/kg/min). Isosorbide dinitrate 0.5 µg/kg/min was infused in all patients throughout the study period. The patients' lungs were ventilated with a tidal volume of 8-10 ml/kg, I : E ratio of 1 : 2, at a rate of 8-12 breaths/min with 40% oxygen in air and positive end-expiratory pressure of 5 cmH2O during the surgery. After induction of anesthesia, a transesophageal echocardiography probe was inserted to assess global cardiac function and detect newly developing segmental wall motion abnormalities. Intravascular volume replacement was managed with crystalloid and colloid solutions to maintain the pulmonary capillary wedge pressure between 8 and 16 mmHg, according to the baseline values. During the period of heart displacement, crystalloid solution was infused at a fixed rate of 6-8 ml/kg, whereas colloid solution was infused to compensate for the amount of blood loss collected by a cell salvage device. The salvaged blood by the cell salvage device was rein fused into the patient before the end of the surgery. Hemodynamic management during the period of heart displacement and grafting was as follows: 1) maintenance of mean systemic arterial pressure above 70 mmHg either with 10-20° Trendelenburg position and/or norepinephrine infusion; 2) infusion of milrinone in patients with SvO2 < 60% for longer than 10 min and/or development of mitral regurgitation ≥ grade 3 with concomitant rise in mean pulmonary arterial pressure >30 mmHg. Allogenic packed red blood cells were transfused when the hematocrit level was <25% throughout the study period. Central temperature measured by PAC was maintained above 36℃ with a warm mattress, forced warm-air blanket, and fluid warmer as necessary.
All surgical procedures were performed by one surgeon through a median sternotomy, and the heart was displaced using posterior pericardial stitch, large (12 × 70 cm) gauze swabs, and tissue stabilizer (Rosta® 2.0, Yoorim Corporation, Chungbuk, Korea). The sequence of grafting was always the left internal mammary artery to left anterior descending coronary artery (LAD) first, followed by grafting on the circumflex coronary artery and the right coronary artery (RCA) by way of composite Y graft consisting of radial artery or saphenous vein with left internal mammary artery and/or by use of right internal mammary artery as necessary. An intracoronary shunt was used during grafting procedures on the LAD and distal RCA. All patients were transferred to the intensive care unit (ICU) after surgery.
Assessed preoperative data included demographic data, serum creatinine (Cr) level, troponin (Tn)-T level, cardiac medications, and presence of diabetes mellitus, hypertension, chronic renal failure, recent MI (within one month), left main disease, and the percentage of stenosis of the coronary arteries. Assessed operative data included duration of surgery, number of grafts performed, and graft reconstruction time.
In the ICU and during the period of postoperative hospitalization, the following variables in conjunction with five major morbidity endpoints were assessed and recorded: permanent stroke, renal dysfunction, hemostatic re-exploration, deep sternal wound infection, number of patients requiring prolonged ventilation (>48 h), as well as time to extubation and length of stay in the ICU [22]. For clarification, renal dysfunction was defined as acute postoperative renal insufficiency resulting in one or more of the following: (1) increase of Cr to >2.0 mg/dl, (2) ≥50% increase in Cr over preoperative baseline value, or (3) new requirement for dialysis. Tn-T was measured upon arrival at the ICU, and every 8 h until 24 h after surgery. Postoperative MI was defined as the occurrence of increase in Tn-T ≥ 0.5 ng/ml (five times above the upper normal limit) and development of new pathologic Q wave or new left bundle branch block [23]. Decisions for extubation and discharge from ICU were made at the discretion of the ICU staff, consisting of cardiothoracic surgeons and anesthesiologists not aware of this study, according to the standard ICU protocols of our institution. Criteria for weaning from ventilator support included an appropriate sensorium, hemodynamic stability (CI > 2.2 L/min/m2; mean arterial pressure >60 mmHg; pulmonary artery diastolic pressure <20 mmHg; and no significant arrhythmias); PaO2 (arterial partial pressure of oxygen) > 100 mmHg with a FiO2 (fraction of inspired oxygen) = 0.4; minimal chest tube drainage; urine output 0.5 ml/kg/h; and temperature > 35.5℃. Discharge criteria from the ICU were as follows: stabilized patient's clinical status without the need for ICU monitoring and care (which includes no further requirement for either inotropic or vasoactive agents except norepinephrine infusion less than 0.05 µg/kg/min), and no plan for further active intervention.
Statistical analyses were performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as the number of patients or mean ± SD. Data between the groups were compared using the Chi-square test, Fisher's exact test, or independent t-test as appropriate. A P value of less than 0.05 was considered statistically significant.
Go to : 
Patients' characteristics and operative data are shown in Table 1. Patients' characteristics including the existence of left main coronary artery disease and degree of coronary stenosis were all similar between the groups. There was, however, a trend toward higher incidence of recent MI in the statin group (24 vs. 34, P = 0.088). Operative characteristics including the number of grafts and graft reconstruction time were also all similar.
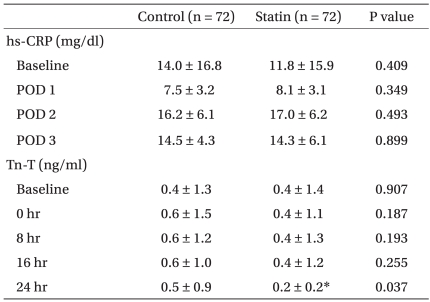
Postoperative data and biochemical data are shown in Tables 2 and 3, respectively. Among the major morbidity endpoints, there was a trend toward higher incidence of renal dysfunction in the control group, with significantly more patients requiring dialysis (Table 2). The hs-CRP values measured at various time points were all similar between the groups. Tn-T measured at predefined time points were also all similar, except at 24 h after surgery, when it was significantly lower in the statin group compared to the control group (Table 3).
Go to : 
In the current trial addressing the effects of preoperative statin therapy on myocardial protection and morbidity endpoints in patients with elevated hs-CRP undergoing OPCAB, we could observe beneficial effects in terms of less Tn-T release and less incidence of new requirements for hemodialysis in patients treated with statins.
As understanding of the pathophysiology of atherosclerosis has evolved, statins' beneficial effects on the cardiovascular system came to include concept of the anti-inflammatory effect, improvement of endothelial dysfunction, increased nitric oxide bioavailability, antioxidant properties, plaque stabilization, inhibition of thrombosis, and smooth muscle proliferation, as well as the lipid-lowering effect [4,5,8]. According to recent AFCAPS/TexCAPS research [7], statin therapy successfully reduced the incidence of MI, unstable angina, and coronary revascularization, regardless of cholesterol levels. Subsequently, statins are being widely prescribed for patients with a normal range of cholesterol levels to prevent acute coronary events. Several studies demonstrated that statin therapy is beneficial in patients undergoing variable cardiothoracic surgery [9]. Although conflicting results have been reported, many studies demonstrated that statins improve bypass graft patency rates, and reduce perioperative and long-term mortality rates, revascularization rates, and the number of postoperative complications and hospital stays in patients undergoing on-pump CABG [9-15].
The hs-CRP, a well-known marker of severity of coronary artery disease and a relevant predictor of outcome in patients with heart failure, has become a target of statin therapy because stains reduce CRP levels independently of their effects on cholesterol. Moreover, preoperative hs-CRP has been shown to be closely associated with early graft occlusion, cardiovascular events, and overall survival after CABG [17-19]. Multivessel off-pump CABG requires mechanical heart displacement accompanied by cumulative, regional, and warm myocardial ischemia-reperfusion injury, and is associated with a considerable degree of inflammatory response [24-26]. Thus, patients with elevated hs-CRP undergoing CABG are considered to derive the most benefits from statin therapy, and we tried to assess the effect of statin therapy on myocardial protection and morbidity endpoints in patients with elevated hs-CRP undergoing off-pump CABG.
Of interest to note was that Tn-T level at 24 h after surgery was significantly lower in the patients who had taken any statins for at least one week before surgery despite their higher trend of recent preoperative MI history. We did not observe any significant increase in postoperative Tn-T values in both groups compared to each baseline value, which may be attributable to the high prevalence of recent MI (40%) and the already elevated baseline Tn-T levels in both groups. However, a trend could be seen in the control group toward a minor increase in Tn-T values as time passed, whereas it remained essentially the same until 16 hr postoperatively in the statin group, with a trend toward decreasing at 24 hr postoperatively. Considering the nearly absolute myocardial tissue specificity and the high predictive value of Tn-T for perioperative myocardial ischemia after CABG [26,27], our result indicates preoperative statin use could reduce the severity of postoperative myocardial necrosis and protect the myocardium after off-pump CABG in patients whose preoperative hs-CRP level is elevated.
With regard to the major morbidity endpoints, we did not observe significant differences between the groups. This is in accordance with the results of a single center retrospective study on 1,706 patients undergoing CABG in which preoperative statin use was not associated with a reduction of perioperative MI, stroke, or prolonged ventilation [9]. However, our patients on statins displayed a trend toward lower renal dysfunction, and significantly fewer subjects required dialysis after surgery. This is supported by another retrospective cohort study of patients undergoing CABG, which demonstrated 50% lower incidence of new postoperative renal insufficiency, suggesting the renoprotective effect of preoperative statin therapy [28]. The relationship between dyslipidemia and renal function was well demonstrated, and other complex actions of statins, including restoration of endothelium-dependent NO production, anti-inflammatory process, and improvement of microvascular circulation, are supposed to be the mechanisms of statins' renoprotective effects [3,9].
Contrary to the result of a study that demonstrated reduced postoperative CRP concentrations following loading doses of atorvastatin in patients undergoing on-pump CABG [29], we found no relationship between preoperative statin therapy and postoperative hs-CRP levels. This may be attributable to heterogeneous types and dosages of statin therapy, as well as to the different nature of inflammatory response with regard to the degree and kinetics by the avoidance of cardiopulmonary bypass [22,30].
The limitations of this study are as follows. Being a retrospective study, patients received statin therapy at the discretion of the attending physician and not according to randomization. Despite careful adjustment for potential confounders that might affect results, immeasurable confounding factors may still exist. Secondly, we defined the statin group as the patients who had taken statins for at least one week to allow for a steady state of drug concentrations according to the pharmacokinetic properties of the currently used statins; however, there is no confirmed duration of taking statins to show their effectiveness. Since the patients' characteristics and operative data were all similar, and because all patients received standard anesthetic and surgical care according to institutional guidelines, the bias should be minimal. Thus, our results should provide novel evidence to support the beneficial effects of preoperative statin therapy in patients undergoing multivessel off-pump CABG. Based on these results, further prospective, randomized, and controlled trials should be performed to obtain definite conclusions.
In conclusion, we observed beneficial effects of preoperative statin therapy for at least one week in terms of less myocardial enzyme release and fewer patients requiring dialysis following off-pump CABG in patients whose preoperative hs-CRP was elevated.
Go to : 
References
1. Collins R, Armitage J, Parish S, Sleigh P, Peto R. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003; 361:2005–2016. PMID: 12814710.
2. Chan AW, Bhatt DL, Chew DP, Quinn MJ, Moliterno DJ, Topol EJ, et al. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation. 2002; 105:691–696. PMID: 11839623.

3. Youssef F, Gupta P, Seifalian AM, Myint F, Mikhailidis DP, Hamilton G. The effect of short term treatment with simvastatin on renal function in patients with peripheral arterial disease. Angiology. 2004; 55:53–62. PMID: 14759090.
4. Almuti K, Rimawi R, Spevack D, Ostfeld RJ. Effects of statins beyond lipid lowering: potential for clinical benefits. Int J Cardiol. 2006; 109:7–15. PMID: 16054715.

5. Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004; 109(23 Suppl 1):III39–III43. PMID: 15198965.

6. Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther. 2003; 99:95–112. PMID: 12804701.

7. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998; 279:1615–1622. PMID: 9613910.
8. Paraskevas KI, Tzovaras AA, Briana DD, Mikhailidis DP. Emerging indications for statins: a pluripotent family of agents with several potential applications. Curr Pharm Des. 2007; 13:3622–3636. PMID: 18220799.

9. Paraskevas KI. Applications of statins in cardiothoracic surgery: more than just lipid-lowering. Eur J Cardiothorac Surg. 2008; 33:377–390. PMID: 18248999.

10. Clark LL, Woolsen RF, Crawford FA Jr, Crumbley AJ III, Kratz JM. Preoperative statin treatment is associated with reduced postoperative complications and morbidity in cardiac surgery patients: An 8-year retrospective study. Circulation. 2004; 110(Suppl III):506.
11. Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol. 2000; 86:1128–1130. PMID: 11074212.

12. Christenson JT. Preoperative lipid control with simvastatin reduces the risk for graft failure already 1year after myocardial revascularization. Cardiovasc Surg. 2001; 9:33–43. PMID: 11137806.
13. Dotani MI, Morise AP, Haque R, Jain AC, Gupta N, Gibson CM. Association between short-term simvastatin therapy before coronary artery bypass grafting and postoperative myocardial blood flow as assessed by positron emission tomography. Am J Cardiol. 2003; 91:1107–1109. PMID: 12714156.

14. Collard CD, Body SC, Shernan SK, Wang S, Mangano DT. Preoperative statin therapy is associated with reduced cardiac mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2006; 132:392–400. PMID: 16872968.

15. Lazar HL. Role of statin therapy in the coronary bypass patient. Ann Thorac Surg. 2004; 78:730–740. PMID: 15276569.

16. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008; 359:2195–2207. PMID: 18997196.

17. Balciunas M, Bagdonaite L, Samalavicius R, Griskevicius L, Vuylsteke A. Pre-operative high sensitive C-reactive protein predicts cardiovascular events after coronary artery bypass grafting surgery: a prospective observational study. Ann Card Anaesth. 2009; 12:127–132. PMID: 19602737.

18. Hedman A, Larsson PT, Alam M, Wallen NH, Nordlander R, Samad BA. CRP, IL-6 and endothelin-1 levels in patients undergoing coronary artery bypass grafting. Do preoperative inflammatory parameters predict early graft occlusion and late cardiovascular events? Int J Cardiol. 2007; 120:108–114. PMID: 17141340.

19. Kangasniemi OP, Biancari F, Luukkonen J, Vuorisalo S, Satta J, Pokela R, et al. Preoperative C-reactive protein is predictive of long-term outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006; 29:983–985. PMID: 16682213.

20. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001; 344:1959–1965. PMID: 11430324.

21. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005; 352:20–28. PMID: 15635109.

22. Shroyer AL, Coombs LP, Peterson ED, Eiken MC, DeLong ER, Chen A, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003; 75:1856–1865. PMID: 12822628.

23. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007; 116:2634–2653. PMID: 17951284.
24. Raja SG, Berg GA. Impact of off-pump coronary artery bypass surgery on systemic inflammation: current best available evidence. J Card Surg. 2007; 22:445–455. PMID: 17803591.

25. Rastan AJ, Bittner HB, Gummert JF, Walther T, Schewick CV, Girdauskas E, et al. On-pump beating heart versus off-pump coronary artery bypass surgery-evidence of pump-induced myocardial injury. Eur J Cardiothorac Surg. 2005; 27:1057–1064. PMID: 15896617.

26. Han SH, Kim JH, Sim SE, Ham BM. Hemodynamic changes during off pump coronary artery bypass surgery. Korean J Anesthesiol. 2002; 43:44–48.

27. Holmvang L, Jurlander B, Rasmussen C, Thiis JJ, Grande P, Clemmensen P. Use of biochemical markers of infarction for diagnosing perioperative myocardial infarction and early graft occlusion after coronary artery bypass surgery. Chest. 2002; 121:103–111. PMID: 11796438.

28. Tabata M, Khalpey Z, Pirundini PA, Byrne ML, Cohn LH, Rawn JD. Renoprotective effect of preoperative statins in coronary artery bypass grfating. Am J Cardiol. 2007; 100:442–444. PMID: 17659925.
29. Krivov N, Adler Z, Saloma R, Hawadie A, Azzam ZS. Targeting C-reactive protein using high-dose atorvastatin before coronary artery bypass graft surgery. Exp Clin Cardiol. 2008; 13:171–174. PMID: 19343161.
30. Tomic V, Russwurm S, Möller E, Claus RA, Blaess M, Brunkhorst F, et al. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation. 2005; 112:2912–2920. PMID: 16275880.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download