Abstract
Rhabdomyolysis is a rare but potentially lethal clinical syndrome that results from acute muscle fiber necrosis with leakage of muscle constituents into blood. This devastating disease could be due to muscle compression caused by urologic positioning for a lengthy nephrectomy. In this regard, laparoscopic renal surgery may be a risk for the development of rhabdomyolysis. This phenomenon of massive muscle necrosis can produce secondary acute renal failure. The risk factors have to be managed carefully during anesthetic management. Here, we report a case of a patient with rhabdomyolysis that developed in the flexed lateral decubitus position during laparoscopic nephrectomy.
Go to : 
Rhabdomyolysis is a disease that results from the acute injury of skeletal muscle with leakage of muscle constituents into blood and extracellular tissue [1]. It can, though rarely does, occur in various diseases of destruction of muscle membranes, including muscle strain, persistent coma such as with carbon monoxide poisoning, drug or alcohol abuse, connective tissue disease, genetic disease, and excessive exercise [2]. Rhabdomyolysis can also be caused by compression of muscle due to operative positioning during prolonged surgery [1,3]. We report a case of a patient with rhabdomyolysis following laparoscopic radical nephrectomy under general anesthesia.
A 51-year-old man (height 183 cm, weight 96 kg) was admitted for radical nephrectomy with the diagnosis of renal cell carcinoma. A CT scan demonstrated a 7.3 cm right lower pole enhancing hyperemic renal mass. He had undergone surgery for varicose veins under general anesthesia 3 months prior; his tobacco use was 1 pack/day for 30 years and alcohol consumption was soju 1 bottle/day, 3-4 days/week. A preoperative chest x-ray revealed multiple hematogenous metastatic nodules and emphysema on both lung fields. He had no history of dyspnea and no wheezing sounds on his lung exam, however. ECG was within normal range. Postoperative chemotherapy was planned for metastatic lung cancer. Preoperative laboratory results were the following: hemoglobin was 13.5 g/dl, hematocrit was 42.3%, AST/ALT were 10/20 IU, and BUN/creatinine were 12.8/0.8 mg/dl. His preoperative blood pressure was 120/80 mmHg and heart rate was 72 beats/minute.
After arriving at the operating theater, ECG, noninvasive blood pressure measurement, pulse oximeter, and capnography were applied. Initial vital signs in the operation room were blood pressure 110/73 mmHg, heart rate 65 beats/min, respiratory rate 18 breaths/min. For the induction of anesthesia, propofol 120 mg and rocuronium bromide 50 mg were injected intravenously, and 6 L/min of O2 and desflurane were inhaled. Anesthesia was maintained with O2 and N2O (FiO2 50%), desflurane 5.0-7.0%, and an intravenous target controlled infusion of remifentanil with a target of 2.0-3.0 ng/ml. After arterial catheterization, the patient was placed in a lateral decubitus position with a kidney rest on the lower part of the iliac crest. The operation table was flexed by 30 degrees, and the patient's buttocks were fastened with a hip strap, with careful attention paid to prevent the femur head from encountering pressure. After a trocar was inserted via a retroperitoneal approach, pneumoperitoneum was produced until the abdominal insufflation pressure was 11 mmHg, and 3 port laparoscopic radical nephrectomy was performed. There was no change in vital signs at initiation of the surgery, and vital signs during surgery were maintained, with blood pressure 90-100/55-65 mmHg, heart rate 60-75 beats/min, respiratory rate 12-15 breaths/min, peak inspiration pressure 18 cmH2O, end tidal CO2 38 mmHg, and SaO2 97%. Two branches of the right renal artery and one branch of the right renal vein were ligated.
Four hours after initiation of surgery, the operating procedure was changed to open nephrectomy, because one branch of the right renal artery entered into the back of the kidney. After ligation of the right renal artery, the right kidney and the oncologic mass were resected, and the peritoneal wall was closed. During the operation, an arterial blood gas analysis was performed and hemoglobin, hematocrit, and electrolytes were checked. The results were within normal range. At one hour and 20 minutes after laparotomy, labs showed a hemoglobin level of 7.8 g/ml and hematocrit of 23.0%, so 420 ml of packed red blood cells were transfused. The mean operative time was 7 hours, and blood loss and urine output were 800 ml and 400 ml, respectively. The total amounts of fluids administered were 2,100 ml of crystalloid solution and 500 ml of colloid solution. After reversing muscle relaxation and making sure the patient had an adequate amount of spontaneous breathing, the endotracheal tube was removed.
The patient was transported to the postanesthetic care unit. After awakening, he complained of back pain on the left side. Examination showed slight redness on his flank that extended to the buttocks, but localized swelling and warmth were not observed. Due to the postoperative low back pain, patient control analgesia (PCA) was administered in doses of 1 ml. An arterial blood gas analysis was performed while the patient was spontaneously breathing with mask oxygenation at 8 L/min. The results of the blood gas analysis showed a pH of 7.40, PaCO2 of 43.1 mmHg, PaO2 of 98 mmHg, and HCO3- of 22.6 mmol/L. His vital signs were stable, and the patient was transferred to the ICU.
On postoperative day 1, laboratory tests revealed AST/ALT of 497/175 IU, BUN/creatinine of 27/3.5 mg/dl, creatinine phospokinase (CPK) of 21,817 IU/L, and lactate dehydrogenase (LDH) of 1,973 IU/L. The patient's urine output decreased to 83 ml during 24 hours and did not respond to diuretics. On postoperative day 2, the patient had persistent pain in the area of his left flank, and laboratory tests revealed AST/ALT of 432/176 IU, BUN/creatinine of 40.9/5.1 mg/dl, and his urine output was still low at 254 ml over 24 hours.
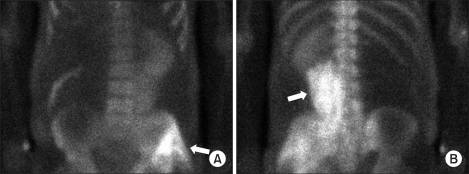
Renal ultrasonography results did not show any left kidney abnormality, such as pelvicouretral dilatation indicating hydronephrosis or obstruction. On postoperative day 3, BUN/creatinine increased to 51/6.8 mg/dl, and urine output was less than 10 ml. At that point, the patient underwent hemodialysis under the diagnosis of anuric acute renal failure. On postoperative day 4, CPK was 7,091 IU/L, LDH was 2,161 IU/L, and serum myoglobin was more than 3,000 ng/ml. A bone scan showed myolysis of the psoas and gluteus muscles (Fig. 1). On postoperative day 6, aldose was 43.2 U/L, and the patient had persistent pain of the left flank. On postoperative day 8, BUN/creatinine level increased to 50/10.8 mg/dl and then gradually decreased. On postoperative day 20, BUN/creatinine level decreased to 23/2.0 mg/dl and continued to drop, and urine output returned to normal. CPK and LDH levels also dropped to 127 IU/L and 572 IU/L on postoperative day 15. Hemodialysis was performed 12 times over 2 weeks.
Once pain management was achieved, the patient was transferred to the ward. He was discharged 41 days after surgery and has had no problems since that time.
Go to : 
Rhabdomyolysis can be defined as a reversible or irreversible injury due to degeneration of the cell membrane or an injury to the sarcolemma of skeletal muscle, resulting in leakage of its components into the blood or extracellular environment [1-4]. Injury to the sarcolemma may be caused by hypoxia, reperfusion injury, or direct injury to the cell membrane, causing leakage of intracellular proteins into the extracellular environment. Leaked substances include CPK, LDH, myoglobin, aldose, phosphate, and potassium, resulting in increased plasma concentrations of these substances. Criteria for the diagnosis of rhabdomyolysis includes the absence of myocardial ischemia, five times more than normal CPK levels (greater than 1,000), or presence of myoglobinuria [2,4]. Rhabdomyolysis occurs more frequently in males, as in this case, and is prevalent in patients in their late 40s. Rhabdomyolysis after laparoscopic nephrectomy occurs in 0.4 to 4.9% of surgeries. Postoperative rhabdomyolysis is known to be associated with ruptured abdominal aortic aneurysm surgery, revascularization of vascular occlusion, and urology surgery [5,6]. Risk factors include male gender, high body mass index, prolonged surgery, lateral decubitus position, and flexed lateral decubitus position. Additional risk factors include kidney disease, extracellular dehydration, and hypoperfusion of the kidney [6]. The most common sites include the muscles around the lumbar spine and gluteus, as in this case [7-9].
Symptoms of rhabdomyolysis include pain, tenderness, edema, weakness of the affected muscles, and muscle pain related to passive stretch [5]. Rhabdomyolysis can be diagnosed by the presence of the myoglobinuria. Myoglobinuria occurs when myoglobin levels in plasma exceed 0.5-1.5 mg/dl and is characterized by dark brown urine. In some cases of rhabdomyolysis, however, myoglobinuria intermittently occurs and thus myoglobin cannot be detected in the urine [6,9,10]. Urine may not test positive for myoglobin because these proteins are metabolized to bilirubin, excreted quickly and appear only temporarily in the urine. In this case, we could not grossly detect myoglobinuria, mostly likely because we could not distinguish the characteristic dark brown color of myoglobinuria from postoperative hematuria. Obtaining the patient's serum myoglobin level could not have been useful, because serum myoglobin can be increased and normalized in a short period of time. The CPK level is known to be the most sensitive test for rhabdomyolysis, because it is increased in proportion to the amount of muscle damage [2,4]. In this case, we measured CPK, LDH, AST, and ALT on a daily basis and monitored the patient's progress.
Complications of rhabdomyolysis include persistent muscle weakness, atrophy, and nerve dysfunction, with acute renal failure as the most serious complication [11]. Acute renal failure occurs in 15-46% of cases of rhabdomyolysis and death occurs in 3.4% of cases [12]. The mechanism of acute renal failure in patients with rhabdomyolysis is not clearly known, but it is thought to be a complex mechanism that includes myoglobin-induced vasoconstriction, cytotoxicity of the heme molecule, and blockage of renal tubules with precipitated myoglobin that results in reverse pressure [4,5]. Dehydration and acidosis as a result of fluid loss are also a part of this phenomenon [5].
The flexed lateral decubitus position, called the kidney position or nephrectomy position, is a position commonly used in urological surgery [13,14]. Targa et al. [3] studied the association between rhabdomyolysis and operative position and concluded that rhabdomyolysis is associated with the lateral position and long lasting surgery. Glassman et al. [6] suggested the possibility that placing a patient in a rotated supine position instead of the flexed lateral position would reduce the occurrence of rhabdomyolysis. It has also been suggested that intraperitoneal CO2 infusion for laparoscopic surgery resulting in abdominal pressure of 12-15 mmHg can further add to low perfusion pressure among the obese, so it is possible that in our case laparoscopic surgery served as one of the factors causing low perfusion.
Permissive hypertension would provide improved perfusion of compressed tissue and prevent the development of rhabdomyolysis [6]. In general, surgeons want to establish a blood pressure that is low enough to prevent blood from disturbing the operative field and avoid fluid overload, which could cause postoperative pulmonary edema. Laparoscopic surgery and low blood pressure might be precipitating factors for rhabdomyolysis, however, because they perturb perfusion to muscles under pressure.
In the anesthesiologist's view, the disease to distinguish from postoperative rhabdomyolysis is malignant hyperthermia [1,15]. In this case, increased CO2 production, which is the earliest and most sensitive indicator of malignant hyperthermia, was not observed and the body temperature was normal. Also, there was no rigidity of the masseter muscle and no tachycardia, arrhythmia, and tachypnea, signs that are known to occur within 5 minutes of induction of anesthesia in malignant hyperthermia. Finally, the patient's CPK level was higher than those of other hyperthermia cases reported in the country. In accordance with these facts, the diagnosis of malignant hyperthermia was excluded [12,15].
Treatment of rhabdomyolysis includes aggressive fluid resuscitation with central venous pressure monitoring, use of mannitol, and infusion of sodium bicarbonate to alkalinize the urine and stop the progression of renal failure. When renal failure occurs and it is indicated, hemodialysis is important to recover renal function [4,5,9]. The criteria for hemodialysis in patients with rhabdomyolysis includes serum creatinine more than 1.5 mg/dl, base deficit less than -4, serum CPK more than 5,000 IU/L, and the presence of myoglobinuria. Sharp et al. [4] reported that renal failure requiring dialysis occurs in 9.5% of patients with postoperative rhabdomyolysis and that mortality is 3 times higher in patients with rhabdomyolysis who need hemodialysis than in those who do not. Also, it is known that muscle swelling can delay muscle ischemia and cause a second surge of CPK, so vigilance must not be relaxed after the initial treatment [2]. If patients have surgery again at a later date, anesthesiologists should proceed with care, because rhabdomyolysis reoccurs in 11% of patients [2].
In conclusion, it is important to note that anesthesiologists should pay attention to positioning during surgery, because prevention methods are critical to reduce the risk of rhabdomyolysis. That is, appropriate pads on body parts under pressure should be installed during surgery and lowering the kidney rest could be effective even before the end of the operation [5]. More study is needed on the efficacy of devices that measure abdominal pressure; studies are also needed to test how well commercial products like vacuum bean bags or gel cushioning pads, which are designed to distribute the pressure of large areas, can prevent excessive local pressure [6]. In rhabdomyolysis, stable hemodynamic status should be maintained, because hypotension and acidosis may act as contributing factors during anesthesia. If possible, intentional hypertension should be tried in patients who are at risk for rhabdomyolysis. Reduction of operating time by surgeons and interruption of surgery at regular intervals to normalize flexed areas might help prevent rhabdomyolysis. In patients with the possibility of postoperative rhabdomyolysis, it is important to observe the patient's progress and have an early suspicion of rhabdomyolysis, especially when the patient's pain is out of proportion to the clinical situation and skin symptoms and an abnormal urine color are found.
Go to : 
References
1. Ziser A, Friedhoff RJ, Rose SH. Prone position: visceral hypoperfusion and rhabdomyolysis. Anesth Analg. 1996; 82:412–415. PMID: 8561351.
2. Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore). 2005; 84:377–385. PMID: 16267412.
3. Targa L, Droghetti L, Caggese G, Zatelli R, Roccella P. Rhabdomyolysis and operating position. Anaesthesia. 1991; 46:141–143. PMID: 1872430.

4. Sharp LS, Rozycki GS, Feliciano DV. Rhabdomyolysis and secondary renal failure in critically ill surgical patients. Am J Surg. 2004; 188:801–806. PMID: 15619503.

5. Nimmo GR, Lambie AT, Cumming AD. Rhabdomyolysis and acute renal failure. Intensive Care Med. 1989; 15:486–487. PMID: 2607034.

6. Glassman DT, Merriam WG, Trabulsi EJ, Byrne D, Gomella L. Rhabdomyolysis after laparoscopic nephrectomy. JSLS. 2007; 11:432–437. PMID: 18237506.
7. Petit P, Atri M, Rosenthall L, Bret PM, Senterman MK. Computed tomographic detection of skeletal muscle calcifications in rhabdomyolysis. Can Assoc Radiol J. 1992; 43:443–446. PMID: 1450975.
8. Vukanovic S, Hauser H, Curati WL. Myonecrosis induced by drug overdose: pathogenesis, clinical aspects and radiological manifestations. Eur J Radiol. 1983; 3:314–318. PMID: 6653563.
9. Nimmo GR, Stewart SM, English PJ. Myoglobinuric acute renal failure associated with major urologic surgery--an avoidable problem. Intensive Care Med. 1988; 14:244–245. PMID: 3379187.
10. Kim HY, Choi SO, Shin SJ, Kim YK, Han BG, Park SJ, et al. Analysis of 250 cases of rhabdomyolysis. Korean J Nephrol. 1994; 13:810–817.
11. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002; 65:907–912. PMID: 11898964.
12. Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988; 148:1553–1557. PMID: 3382301.

13. Lawson NW, Meyer DJ. Martin JT, Warner MA, editors. Lateral position. Positioning in anesthesia and surgery. 1987. 3rd ed. Philadelphia: W.B. Saunders company;p. 132–146.
14. Dahlberg PJ, Howard RS. Rhabdomyolysis: an unusual postoperative complication. J Urol. 1982; 127:520–521. PMID: 7062429.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download